Self-Organized Nuclear Positioning Synchronizes the Cell Cycle in Drosophila Embryos
- PMID: 30982601
- PMCID: PMC6499673
- DOI: 10.1016/j.cell.2019.03.007
Self-Organized Nuclear Positioning Synchronizes the Cell Cycle in Drosophila Embryos
Abstract
The synchronous cleavage divisions of early embryogenesis require coordination of the cell-cycle oscillator, the dynamics of the cytoskeleton, and the cytoplasm. Yet, it remains unclear how spatially restricted biochemical signals are integrated with physical properties of the embryo to generate collective dynamics. Here, we show that synchronization of the cell cycle in Drosophila embryos requires accurate nuclear positioning, which is regulated by the cell-cycle oscillator through cortical contractility and cytoplasmic flows. We demonstrate that biochemical oscillations are initiated by local Cdk1 inactivation and spread through the activity of phosphatase PP1 to generate cortical myosin II gradients. These gradients cause cortical and cytoplasmic flows that control proper nuclear positioning. Perturbations of PP1 activity and optogenetic manipulations of cortical actomyosin disrupt nuclear spreading, resulting in loss of cell-cycle synchrony. We conclude that mitotic synchrony is established by a self-organized mechanism that integrates the cell-cycle oscillator and embryo mechanics.
Keywords: actomyosin network; cell cycle; collective dynamics; cortical contractility; cytoplasmic flows; embryonic development; nuclear positioning; optogenetics; self-organization; synchronization.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures


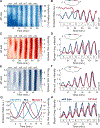


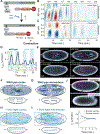

Comment in
-
Nuclear (Bio)physics in the Embryo.Cell. 2019 May 2;177(4):799-801. doi: 10.1016/j.cell.2019.04.031. Cell. 2019. PMID: 31051102
References
-
- Acheson DJ (1990). Elementary fluid dynamics (Clarendon Press; Oxford University Press; ).
-
- Carroll NJ, Jensen KH, Parsa S, Holbrook NM, and Weitz DA (2014). Measurement of flow velocity and inference of liquid viscosity in a microfluidic channel by fluorescence photobleaching. Langmuir 30, 4868–4874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

