AKAP95 Organizes a Nuclear Microdomain to Control Local cAMP for Regulating Nuclear PKA
- PMID: 30982750
- PMCID: PMC6588460
- DOI: 10.1016/j.chembiol.2019.03.003
AKAP95 Organizes a Nuclear Microdomain to Control Local cAMP for Regulating Nuclear PKA
Abstract
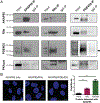
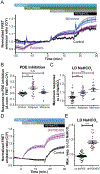
Contrary to the classic model of protein kinase A (PKA) residing outside of the nucleus, we identify a nuclear signaling complex that consists of AKAP95, PKA, and PDE4D5 and show that it forms a functional cyclic AMP (cAMP) signaling microdomain. Locally generated cAMP can accumulate within the vicinity of this complex; however, when cAMP is generated at the plasma membrane, PDE4 serves as a local sink and PDE3 as a barrier to prevent accumulation of cAMP within the microdomain as a means of controlling activation of tethered nuclear PKA.
Keywords: FRET biosensors; compartmentalization; imaging; spatiotemporal.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures


References
-
- Akileswaran L, Taraska JW, Sayer JA, Gettemy JM, and Coghlan VM (2001). A-kinase-anchoring Protein AKAP95 Is Targeted to the Nuclear Matrix and Associates with p68 RNA Helicase. J. Biol. Chem 276, 17448–17454. - PubMed
-
- Arsenijevic T, Degraef C, Dumont JE, Roger PP, and Pirson I (2006). G1/S cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle 5, 1217–1222. - PubMed
-
- Asirvatham AL, Galligan SG, Schillace RV, Davey MP, Vasta V, Beavo JA, and Carr DW (2004). A-Kinase Anchoring Proteins Interact with Phosphodiesterases in T Lymphocyte Cell Lines. J. Immunol - PubMed
-
- Banaszynski LA, Liu CW, and Wandless TJ (2005). Characterization of the FKBP-rapamycin-FRB ternary complex. J. Am. Chem. Soc 127, 4715–4721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

