Tspan8 is expressed in breast cancer and regulates E-cadherin/catenin signalling and metastasis accompanied by increased circulating extracellular vesicles
- PMID: 30982971
- PMCID: PMC6771825
- DOI: 10.1002/path.5281
Tspan8 is expressed in breast cancer and regulates E-cadherin/catenin signalling and metastasis accompanied by increased circulating extracellular vesicles
Abstract
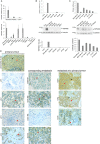
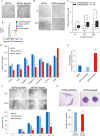
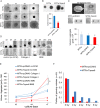
Tspan8 exhibits a functional role in many cancer types including pancreatic, colorectal, oesophagus carcinoma, and melanoma. We present a first study on the expression and function of Tspan8 in breast cancer. Tspan8 protein was present in the majority of human primary breast cancer lesions and metastases in the brain, bone, lung, and liver. In a syngeneic rat breast cancer model, Tspan8+ tumours formed multiple liver and spleen metastases, while Tspan8- tumours exhibited a significantly diminished ability to metastasise, indicating a role of Tspan8 in metastases. Addressing the underlying molecular mechanisms, we discovered that Tspan8 can mediate up-regulation of E-cadherin and down-regulation of Twist, p120-catenin, and β-catenin target genes accompanied by the change of cell phenotype, resembling the mesenchymal-epithelial transition. Furthermore, Tspan8+ cells exhibited enhanced cell-cell adhesion, diminished motility, and decreased sensitivity to irradiation. As a regulator of the content and function of extracellular vesicles (EVs), Tspan8 mediated a several-fold increase in EV number in cell culture and the circulation of tumour-bearing animals. We observed increased protein levels of E-cadherin and p120-catenin in these EVs; furthermore, Tspan8 and p120-catenin were co-immunoprecipitated, indicating that they may interact with each other. Altogether, our findings show the presence of Tspan8 in breast cancer primary lesion and metastases and indicate its role as a regulator of cell behaviour and EV release in breast cancer. © 2019 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: Tspan8; beta-catenin signalling pathway; breast cancer; extracellular vesicles; mesenchymal-epithelial transition; metastases; tetraspanins; three-dimensional cell culture.
© 2019 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

