A Multicenter, Randomized, Placebo-Controlled Trial of Atorvastatin for the Primary Prevention of Cardiovascular Events in Patients With Rheumatoid Arthritis
- PMID: 30983166
- PMCID: PMC6771601
- DOI: 10.1002/art.40892
A Multicenter, Randomized, Placebo-Controlled Trial of Atorvastatin for the Primary Prevention of Cardiovascular Events in Patients With Rheumatoid Arthritis
Abstract
Objective: Rheumatoid arthritis (RA) is associated with increased cardiovascular event (CVE) risk. The impact of statins in RA is not established. We assessed whether atorvastatin is superior to placebo for the primary prevention of CVEs in RA patients.
Methods: A randomized, double-blind, placebo-controlled trial was designed to detect a 32% CVE risk reduction based on an estimated 1.6% per annum event rate with 80% power at P < 0.05. RA patients age >50 years or with a disease duration of >10 years who did not have clinical atherosclerosis, diabetes, or myopathy received atorvastatin 40 mg daily or matching placebo. The primary end point was a composite of cardiovascular death, myocardial infarction, stroke, transient ischemic attack, or any arterial revascularization. Secondary and tertiary end points included plasma lipids and safety.
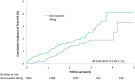
Results: A total of 3,002 patients (mean age 61 years; 74% female) were followed up for a median of 2.51 years (interquartile range [IQR] 1.90, 3.49 years) (7,827 patient-years). The study was terminated early due to a lower than expected event rate (0.70% per annum). Of the 1,504 patients receiving atorvastatin, 24 (1.6%) experienced a primary end point, compared with 36 (2.4%) of the 1,498 receiving placebo (hazard ratio [HR] 0.66 [95% confidence interval (95% CI) 0.39, 1.11]; P = 0.115 and adjusted HR 0.60 [95% CI 0.32, 1.15]; P = 0.127). At trial end, patients receiving atorvastatin had a mean ± SD low-density lipoprotein (LDL) cholesterol level 0.77 ± 0.04 mmoles/liter lower than those receiving placebo (P < 0.0001). C-reactive protein level was also significantly lower in the atorvastatin group than the placebo group (median 2.59 mg/liter [IQR 0.94, 6.08] versus 3.60 mg/liter [IQR 1.47, 7.49]; P < 0.0001). CVE risk reduction per mmole/liter reduction in LDL cholesterol was 42% (95% CI -14%, 70%). The rates of adverse events in the atorvastatin group (n = 298 [19.8%]) and placebo group (n = 292 [19.5%]) were similar.
Conclusion: Atorvastatin 40 mg daily is safe and results in a significantly greater reduction of LDL cholesterol level than placebo in patients with RA. The 34% CVE risk reduction is consistent with the Cholesterol Treatment Trialists' Collaboration meta-analysis of statin effects in other populations.
© 2019, The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

Comment in
-
Lipids and Cardiovascular Risk Through the Lens of Rheumatoid Arthritis.Arthritis Rheumatol. 2019 Sep;71(9):1393-1395. doi: 10.1002/art.40891. Epub 2019 Jul 22. Arthritis Rheumatol. 2019. PMID: 30983150 No abstract available.
References
-
- Aviña‐Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Rheum 2008;59:1690–7. - PubMed
-
- Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 2011;70:8–14. - PubMed
-
- Radner H, Lesperance T, Accortt NA, Solomon DH. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res (Hoboken) 2017;69:1510–8. - PubMed
-
- Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 2015;11:693–704. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 16514/ARC_/Arthritis Research UK/United Kingdom
- MR/K015346/1/MRC_/Medical Research Council/United Kingdom
- 19704/ARC_/Arthritis Research UK/United Kingdom
- SP/08/010/25939/BHF_/British Heart Foundation/United Kingdom
- MC_UU_12026/5/MRC_/Medical Research Council/United Kingdom
- 19704/VAC_/Versus Arthritis/United Kingdom
- MC_UU_12023/14/MRC_/Medical Research Council/United Kingdom
- SP/06/001/BHF_/British Heart Foundation/United Kingdom
- SP/14/3/31114/BHF_/British Heart Foundation/United Kingdom
- MC_U137686853/MRC_/Medical Research Council/United Kingdom
- MR/P020941/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials

