Mucocutaneous Adverse Reactions of Cancer Chemotherapy and Chemoradiation
- PMID: 30983608
- PMCID: PMC6440193
- DOI: 10.4103/ijd.IJD_129_17
Mucocutaneous Adverse Reactions of Cancer Chemotherapy and Chemoradiation
Abstract
Background: With the introduction of newer anti-cancer agents, the adverse effects have become more rampant which call for concern in the treatment of patients with cancer. Hence, the assessment and management of dermatological adverse effects of anti-cancer therapy have become a significant part of the care of patients with cancer and require proper and close collaboration between the dermatologists and the oncologists.
Aims: To assess the frequency and pattern of mucocutaneous adverse reactions to cancer chemotherapy and chemoradiation and grade them according to their severity and to identify hematological and biochemical changes related to cancer chemotherapy-induced mucocutaneous adverse reactions.
Materials and methods: This was a descriptive study done among 226 patients in an Indian tertiary care hospital, who presented with mucocutaneous adverse reactions to either chemotherapy alone or combination of chemotherapy and radiation to dermatology, medical oncology and radiotherapy outpatient departments. Detailed history and examination were undertaken. Visual analog score (VAS) was employed to quantify pain and pruritus. Correlation of various biochemical and hematological parameters with chemotherapy-induced adverse reactions was attempted and grading of adverse reactions was done based on the severity scale of Common Terminology Criteria for Adverse Events (CTCAE).
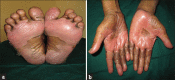
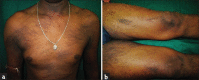
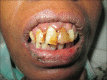
Results: The common cutaneous adverse reactions observed in our study were nail changes (194 patients; 85.84%), followed by skin changes (191; 84.51%), hair changes (159, 70.35%), mucosal changes (34, 15.04%), and other miscellaneous manifestations. Grade 1 manifestations comprised of 49.91% of total manifestations followed by Grade 2 (45.45%) and Grade 3 (5.64%). In addition to bleomycin, other chemotherapeutic agents also had been shown to produce flagellate dermatitis in our study.
Conclusion: Nail changes, skin changes, hair changes and mucosal changes occurred frequently as a significant side effect of chemotherapy, which a physician should be aware of, while selecting a chemotherapeutic drug.
Keywords: Adverse cutaneous reactions; cancer; chemoradiation; chemotherapy.
Conflict of interest statement
There are no conflicts of interest.
Figures



References
-
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45–63. - PubMed
-
- Pavey RA, Kambil SM, Bhat RM. Dermatological adverse reactions to cancer chemotherapy. Indian J Dermatol Venereol Leprol. 2015;81:434. - PubMed
-
- Robert C, Sibaud V, Mateus C, Verschoore M, Charles C, Lanoy E, et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16:e181–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
