Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities?
- PMID: 30983996
- PMCID: PMC6447826
- DOI: 10.3389/fphar.2019.00279
Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities?
Abstract
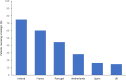
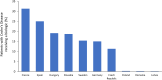
Biological therapies are an effective treatment for a range of immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis, psoriasis, and inflammatory bowel diseases. However, due to their high costs, considerable differences in their utilization exist across the world, even among the various European countries, with many countries restricting access despite professional society guideline recommendations. Adoption of biologics by healthcare providers has been particularly poor in many Central and Eastern European countries. Differences in utilization have also been observed across medical specialties, healthcare providers, and at a regional and national level. The objective of this paper is to provide an overview of the different market access policies for biologics in Europe and to investigate reasons for such differences. One of the potential solutions for providing broader access to IMID patients, where cost is the major barrier, is to encourage the use of biosimilars in place of their reference products. Biosimilars are generally less expensive alternatives to already licensed biological therapies and are approved on the basis that they are similar to the reference product in terms of quality, safety, and efficacy. Budget impact models predict considerable cost savings following the introduction of biosimilars in the next few years. These savings could be used to increase access to biologics and other innovative therapies.
Keywords: biologic; biosimilar; inflammatory bowel disease; patient access; pharmacoeconomics; psoriasis; rheumatoid arthritis; utilization.
Figures

References
-
- Araújo F. C., Fonseca J. E., Goncalves J. (2018). Switching to biosimilars in inflammatory rheumatic conditions: current knowledge. EMJ Rheumatol. 5 66–74.
-
- Atsumi T., Tanaka Y., Yamamoto K., Takeuchi T., Yamanaka H., Ishiguro N., et al. (2017). Clinical benefit of 1-year certolizumab pegol (CZP) add-on therapy to methotrexate treatment in patients with early rheumatoid arthritis was observed following CZP discontinuation: 2-year results of the C-OPERA study, a phase III randomised trial. Ann. Rheum. Dis. 76 1348–1356. 10.1136/annrheumdis-2016-210246 - DOI - PMC - PubMed
-
- Azevedo V., Dörner T., Strohal R., Isaacs J., Castañeda-Hernández G., Gonçalves J., et al. (2017). Biosimilars: considerations for clinical practice. Considerations Med. 1 13–18. 10.1136/conmed-2017-100005 - DOI
Publication types
LinkOut - more resources
Full Text Sources

