Different Oxytocin Responses to Acute Methamphetamine Treatment in Juvenile Female Rats Perinatally Exposed to Stress and/or Methamphetamine Administration
- PMID: 30984017
- PMCID: PMC6447659
- DOI: 10.3389/fphys.2019.00305
Different Oxytocin Responses to Acute Methamphetamine Treatment in Juvenile Female Rats Perinatally Exposed to Stress and/or Methamphetamine Administration
Abstract
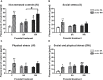
Methamphetamine (MA) is an addictive psychostimulant, often abused by drug-addicted women during pregnancy. The offspring of drug-addicted mothers are often exposed to perinatal stressors. The present study examines the effect of perinatal stressors and drug exposure on plasma oxytocin (OXY) levels in female progeny. Rat mothers were divided into three groups according to drug treatment during pregnancy: intact controls (C); saline (SA, s.c., 1 ml/kg); and MA (s.c., 5 mg/kg). Litters were divided into four groups according to postnatal stressors lasting from PD1 to 21: non-stressed controls (N); maternal separation (S); maternal cold-water stress (W); and maternal separation plus cold-water stress (SW). On postnatal day 30, acute MA or SA was administrated 1 h before the rats were sacrificed. Trunk blood was collected and plasma OXY was measured by specific ELISA after extraction. Our results showed that acute MA administration significantly increases plasma OXY levels in juvenile female rats; this effect was observed in prenatally intact rats only. Prenatal exposure of rats to mild stressor of daily SA injection prevented both acute MA-induced OXY stimulation and also stress-induced OXY inhibition. Although postnatal MA and stress exposure exert opposite effects on OXY release in juvenile rats, our data point out the modulatory role of prenatal mild stress in OXY response to postnatal stressors or MA treatment.
Keywords: maternal separation; methamphetamine; oxytocin; postnatal stress; prenatal stress.
Figures


References
-
- Baracz S. J., Parker L. M., Suraev A. S., Everett N. A., Goodchild A. K., Mcgregor I. S., et al. (2016). Chronic methamphetamine self-administration dysregulates oxytocin plasma levels and oxytocin receptor fibre density in the nucleus accumbens core and subthalamic nucleus of the rat. J. Neuroendocrinol. 28. 10.1111/jne.12337, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

