Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System
- PMID: 30984122
- PMCID: PMC6447710
- DOI: 10.3389/fmicb.2019.00525
Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System
Abstract
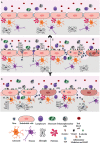
Human infection by different flaviviruses may cause severe neurologic syndromes, through pathogenic mechanisms that are still largely unknown. Japanese encephalitis virus (JEV), West Nile virus (WNV), Zika virus (ZIKV), yellow fever virus (YFV), dengue virus (DENV), and tick-borne encephalitis virus (TBEV) are believed to reach the central nervous system by a hematogenous route, upon crossing the blood-brain barrier. Although the disruption of BBB during flavivirus infection has been largely evidenced in experimental models, the relevance of BBB breakdown for virus entering the brain was not completely elucidated. In vitro models of BBB had demonstrated that these viruses replicated in brain microvascular endothelial cells (BMECs), which induced downregulation of tight junction proteins and increased the permeability of the barrier. Other reports demonstrated that infection of BMECs allowed the basolateral release of infectious particles, without a remarkable cytopathic effect, what might be sufficient for virus invasion. Virus replication and activation of other cells associated to the BBB, mostly astrocytes and microglia, were also reported to affect the endothelial barrier permeability. This event might occur simultaneously or after BMECs infection, being a secondary effect leading to BBB disruption. Importantly, activation of BMECs, astrocytes, and microglia by flaviviruses was associated to the expression and secretion of inflammatory mediators, which are believed to recruit leukocytes to the CNS. The leukocyte infiltrate could further mediate viral invasion through a Trojan horse mechanism and might contribute to BBB breakdown and to neurological alterations. This review discussed the previous studies regarding in vitro and in vivo models of JEV, WNV, ZIKV, YFV, DENV, and TBEV infection and addressed the pathways for BBB overcome and invasion of the CNS described for each virus infection, aiming to increment the knowledge and stimulate further discussion about the role of BBB in the neuropathogenesis of flavivirus infection.
Keywords: Japanese encephalitis virus; West Nile virus; Zika virus; blood-brain barrier; brain microvascular endothelial cells; dengue virus; flavivirus; yellow fever virus.
Figures
References
-
- Araújo F. M., Brilhante R. S., Cavalcanti L. P., Rocha M. F., Cordeiro R. A., Perdigão A. C., et al. . (2011). Detection of the dengue non-structural 1 antigen in cerebral spinal fluid samples using a commercial ayavailable enzyme-linked immunosorbent assay. J. Virol. Methods 177, 128–131. 10.1016/j.jviromet.2011.07.003, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

