Defective Induction of COX-2 Expression by Psoriatic Fibroblasts Promotes Pro-inflammatory Activation of Macrophages
- PMID: 30984165
- PMCID: PMC6448046
- DOI: 10.3389/fimmu.2019.00536
Defective Induction of COX-2 Expression by Psoriatic Fibroblasts Promotes Pro-inflammatory Activation of Macrophages
Abstract
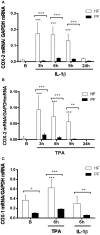
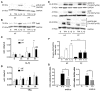
Fibroblasts play an important role as members of the innate immune system through the secretion of COX-2-derived inflammatory mediators such as prostaglandin E2 (PGE2). However, it has been described that dermal fibroblasts behave like mesenchymal stem cells reducing lymphocyte recruitment and dendritic cell activation through PGE2 release. As the role of fibroblasts in psoriasis remains poorly characterized, in the present study we have evaluated the possible influence of PGE2 derived from dermal fibroblasts as modulator of the immune response in psoriatic skin. Our results indicate that under inflammatory conditions, psoriatic fibroblasts showed defective induction of COX-2, which resulted in diminished production of PGE2, in contrast to healthy fibroblasts. This phenotype correlated with deficient c-Jun N-terminal kinase (JNK) activation, in accordance with the hypothesis that alterations in members of the JNK pathway are associated with psoriasis. Furthermore, conditioned medium from psoriatic fibroblasts promoted the polarization of monocytic cells toward a pro-inflammatory profile, effect that was mimicked in healthy fibroblasts after pre-incubation with indomethacin. These results are consistent with a prominent role of dermal fibroblasts in the regulation of inflammatory response through the participation of COX-derived metabolites. This resolutive behavior seems to be defective in psoriatic fibroblasts, offering a possible explanation for the chronification of the disease and for the exacerbation triggered by nonsteroidal anti-inflammatory drugs (NSAIDS) such as indomethacin.
Keywords: cyclooxygenase; fibroblasts; inflammation; macrophages; psoriasis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

