IgA and FcαRI: Pathological Roles and Therapeutic Opportunities
- PMID: 30984170
- PMCID: PMC6448004
- DOI: 10.3389/fimmu.2019.00553
IgA and FcαRI: Pathological Roles and Therapeutic Opportunities
Abstract
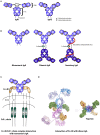
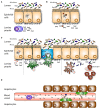
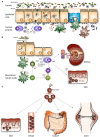
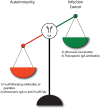
Immunoglobulin A (IgA) is the most abundant antibody class present at mucosal surfaces. The production of IgA exceeds the production of all other antibodies combined, supporting its prominent role in host-pathogen defense. IgA closely interacts with the intestinal microbiota to enhance its diversity, and IgA has a passive protective role via immune exclusion. Additionally, inhibitory ITAMi signaling via the IgA Fc receptor (FcαRI; CD89) by monomeric IgA may play a role in maintaining homeostatic conditions. By contrast, IgA immune complexes (e.g., opsonized pathogens) potently activate immune cells via cross-linking FcαRI, thereby inducing pro-inflammatory responses resulting in elimination of pathogens. The importance of IgA in removal of pathogens is emphasized by the fact that several pathogens developed mechanisms to break down IgA or evade FcαRI-mediated activation of immune cells. Augmented or aberrant presence of IgA immune complexes can result in excessive neutrophil activation, potentially leading to severe tissue damage in multiple inflammatory, or autoimmune diseases. Influencing IgA or FcαRI-mediated functions therefore provides several therapeutic possibilities. On the one hand (passive) IgA vaccination strategies can be developed for protection against infections. Furthermore, IgA monoclonal antibodies that are directed against tumor antigens may be effective as cancer treatment. On the other hand, induction of ITAMi signaling via FcαRI may reduce allergy or inflammation, whereas blocking FcαRI with monoclonal antibodies, or peptides may resolve IgA-induced tissue damage. In this review both (patho)physiological roles as well as therapeutic possibilities of the IgA-FcαRI axis are addressed.
Keywords: CD89; IgA; IgA deficiency; autoimmunity; microbiome; mucosa; therapy; vaccination.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

