Survival in advanced GIST has improved over time and correlates with increased access to post-imatinib tyrosine kinase inhibitors: results from Life Raft Group Registry
- PMID: 30984366
- PMCID: PMC6446260
- DOI: 10.1186/s13569-019-0114-5
Survival in advanced GIST has improved over time and correlates with increased access to post-imatinib tyrosine kinase inhibitors: results from Life Raft Group Registry
Erratum in
-
Correction to: Survival in advanced GIST has improved over time and correlates with increased access to post-imatinib tyrosine kinase inhibitors: results from Life Raft Group Registry.Clin Sarcoma Res. 2019 May 7;9:7. doi: 10.1186/s13569-019-0117-2. eCollection 2019. Clin Sarcoma Res. 2019. PMID: 31080577 Free PMC article.
Abstract
Background: The use of imatinib, sunitinib, and regorafenib has transformed the treatment of advanced GIST. Sunitinib and regorafenib improve progression free-survival in the second (2L) and third (3L) line, respectively, compared with placebo. However, the impact of these agents on overall survival (OS) is unclear.
Methods: The Life Raft Group (LRG) patient registry contains records from 1716 GIST patients; 526 have advanced to at least 2L treatment. Patient-reported treatment and outcome data were examined to determine treatment patterns and their impact on OS.
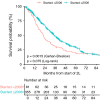
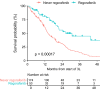
Results: Median OS from start of 2L therapy was 32.4 months for sunitinib (n = 436) compared with 27.1 months for patients treated with any other 2L drug (n = 74, p = 0.023, HR 1.377) and 16.8 months for patients who never received sunitinib in any treatment line (n = 42, p = 0.028, HR 1.52). In patients reporting progression in 2L, the median OS in patients subsequently receiving 3L regorafenib (n = 53, 26.2 months) was longer than that of 3L patients who never received regorafenib in any line of therapy (n = 174, 14.3 months, p = 0.0002, HR 2.231), and was longer than that of patients who received any other 3L treatment (19.8 months, p = 0.044, HR 1.525). OS for advanced GIST patients in the LRG registry has improved over time (p = 0.0013), correlated with the increased use of TKIs in ≥ 2L settings.
Conclusions: In our analysis, sunitinib and regorafenib significantly improved OS compared with patients who never received these agents. Our data also support the hypothesis that the use of KIT/PDGFRA inhibitors, including non-approved agents, has improved OS for patients with imatinib- and sunitinib-resistant GIST.
Keywords: GIST; Gastrointestinal stromal tumors; Regorafenib; Sunitinib; Survival.
Conflict of interest statement
MH: Consulting (Novartis, Bayer, Deciphera Pharmaceuticals, Blueprint Medicines, Molecular MD); Research funding (Deciphera Pharmaceuticals, Blueprint Medicines); Equity interest (Molecular MD); Expert Testimony (Novartis); Patent (1 patent licensed to Novartis). All other authors declare that they have no competing interests.
Figures




References
-
- Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

