Tocopherol biosynthesis in Leishmania (L.) amazonensis promastigotes
- PMID: 30984548
- PMCID: PMC6443866
- DOI: 10.1002/2211-5463.12613
Tocopherol biosynthesis in Leishmania (L.) amazonensis promastigotes
Abstract
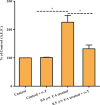
Leishmaniasis is a neglected disease caused by a trypanosomatid protozoan of the genus Leishmania. Most drugs used to treat leishmaniasis are highly toxic, and the emergence of drug-resistant strains has been observed. Therefore, new therapeutic targets against leishmaniasis are required. Several isoprenoid compounds, including dolichols or ubiquinones, have been shown to be important for cell viability and proliferation in various trypanosomatid species. Here, we detected the biosynthesis of tocopherol in Leishmania (L.) amazonensis promastigotes in vitro through metabolic labelling with [1-(n)-3H]-phytol. Subsequently, we confirmed the presence of vitamin E in the parasite by gas chromatography-mass spectrometry. Treatment with usnic acid or nitisinone, inhibitors of precursors of vitamin E synthesis, inhibited growth of the parasite in a concentration-dependent manner. This study provides the first evidence of tocopherol biosynthesis in a trypanosomatid and suggests that inhibitors of the enzyme 4-hydroxyphenylpyruvate dioxygenase may be suitable for use as antileishmanial compounds.
Database: The amino acid sequence of a conserved hypothetical protein [Leishmania mexicana MHOM/GT/2001/U1103] has been deposited in GenBank (CBZ28005.1).
Keywords: Leishmania (L.) amazonensis; isoprenoid; nitisinone, tocopherol; trypanosomatid; usnic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Trinconi CT, Reimao JQ, Coelho AC and Uliana SR (2016) Efficacy of tamoxifen and miltefosine combined therapy for cutaneous leishmaniasis in the murine model of infection with Leishmania amazonensis . J Antimicrob Chemother 71, 1314–1322. - PubMed
-
- Wang KC and Ohnuma S (2000) Isoprenyl diphosphate synthases. Biochim Biophys Acta 1529, 33–48. - PubMed
-
- Urbina JA (1997) Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 114 (Suppl), S91–S99. - PubMed
-
- Pena‐Diaz J, Montalvetti A, Flores CL, Constan A, Hurtado‐Guerrero R, De Souza W, Gancedo C, Ruiz‐Perez LM and Gonzalez‐Pacanowska D (2004) Mitochondrial localization of the mevalonate pathway enzyme 3‐Hydroxy‐3‐methyl‐glutaryl‐CoA reductase in the Trypanosomatidae. Mol Biol Cell 15, 1356–1363. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

