Prolonged Response Induced by Single Agent Vemurafenib in a BRAF V600E Spinal Ganglioglioma: A Case Report and Review of the Literature
- PMID: 30984614
- PMCID: PMC6448025
- DOI: 10.3389/fonc.2019.00177
Prolonged Response Induced by Single Agent Vemurafenib in a BRAF V600E Spinal Ganglioglioma: A Case Report and Review of the Literature
Abstract
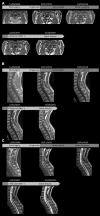
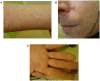
Spinal ganglioglioma is a rare low-grade, slow-growing tumor of the central nervous system affecting mostly children and young adults. After surgery, some patients show tumor recurrence and/or malignant transformation. Gangliogliomas harbor molecular deficiencies such as mutations in the B-rapidly accelerated fibrosarcoma (BRAF) gene, resulting in activation of a downstream signaling pathway and cancer development. Vemurafenib is a BRAF inhibitor used to treat patients with BRAF V600E-mutated cancer. Although a few studies have reported the clinical responses in gangliogliomas, the sequence and duration of treatment have not been established. We describe a case of an adult with a progressive BRAF V600E mutant spinal cord ganglioglioma 9 years after surgery who was treated with vemurafenib. This treatment resulted in a partial response within 2 months, which was sustained for more than a year. The patient then decided to stop treatment because of side effects. Despite this decision, the tumor showed no sign of progression 21 months after treatment discontinuation. This is the first reported case of a response to vemurafenib in an adult with progressive spinal cord BRAF V600E-mutated ganglioglioma which was sustained after treatment discontinuation.
Keywords: BRAF; central nervous system tumor; ganglioglioma; safety; spinal cord; tumor regression; vemurafenib.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

