Construction of a novel phagemid to produce custom DNA origami scaffolds
- PMID: 30984875
- PMCID: PMC6461039
- DOI: 10.1093/synbio/ysy015
Construction of a novel phagemid to produce custom DNA origami scaffolds
Abstract
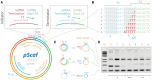
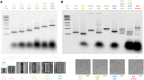
DNA origami, a method for constructing nanoscale objects, relies on a long single strand of DNA to act as the 'scaffold' to template assembly of numerous short DNA oligonucleotide 'staples'. The ability to generate custom scaffold sequences can greatly benefit DNA origami design processes. Custom scaffold sequences can provide better control of the overall size of the final object and better control of low-level structural details, such as locations of specific base pairs within an object. Filamentous bacteriophages and related phagemids can work well as sources of custom scaffold DNA. However, scaffolds derived from phages require inclusion of multi-kilobase DNA sequences in order to grow in host bacteria, and those sequences cannot be altered or removed. These fixed-sequence regions constrain the design possibilities of DNA origami. Here, we report the construction of a novel phagemid, pScaf, to produce scaffolds that have a custom sequence with a much smaller fixed region of 393 bases. We used pScaf to generate new scaffolds ranging in size from 1512 to 10 080 bases and demonstrated their use in various DNA origami shapes and assemblies. We anticipate our pScaf phagemid will enhance development of the DNA origami method and its future applications.
Keywords: DNA nanotechnology; DNA origami; phagemid.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures


References
-
- Rothemund P.W.K. (2006) Folding DNA to create nanoscale shapes and patterns. Nature, 440, 297–302. - PubMed
-
- Specthrie L., Bullitt E., Horiuchi K., Model P., Russel M., Makowski L. (1992) Construction of a microphage variant of filamentous bacteriophage. J. Mol. Biol., 228, 720–724. - PubMed
-
- Pound E., Ashton J.R., Becerril H.A., Woolley A.T. (2009) Polymerase chain reaction based scaffold preparation for the production of thin, branched DNA origami nanostructures of arbitrary sizes. Nano Lett., 9, 4302–4305. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
