Photoelectrocatalytic Arene C-H Amination
- PMID: 30984910
- PMCID: PMC6459354
- DOI: 10.1038/s41929-019-0231-9
Photoelectrocatalytic Arene C-H Amination
Abstract
Photoelectrochemical cells are widely studied for solar energy conversion. However, they have rarely been used for the synthesis of high added-value organic molecules. Here we describe a strategy to use hematite, an abundant and robust photoanode, for non-directed arene C-H amination. Under illumination the photo generated holes in hematite oxidizes electron-rich arenes to radical cations which further react with azoles to give nitrogen heterocycles of medicinal interest. Unusual ortho-selectivity has been achieved probably due to a hydrogen bonding interaction between the substrates and the hexafluoroisopropanol co-solvent. The method exhibits broad scope and is successfully applied for the late-stage functionalization of several pharmaceutical molecules.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures

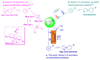



References
-
- Grätzel M. Photoelectrochemical cells. Nature. 2001;414:338. - PubMed
-
- Tachibana Y, Vayssieres L, Durrant JR. Artificial photosynthesis for solar water-splitting. Nat Photonics. 2012;6:511.
-
- Fujishima A, Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature. 1972;238:37. - PubMed
-
- Cha HG, Choi K-S. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nat Chem. 2015;7:328–333. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
