Patient preference for biologic treatments of psoriasis in Japan
- PMID: 30985030
- PMCID: PMC6594072
- DOI: 10.1111/1346-8138.14870
Patient preference for biologic treatments of psoriasis in Japan
Abstract
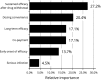
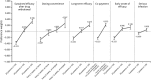
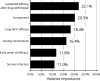
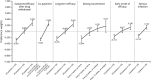
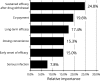
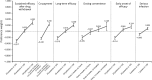
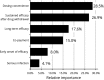
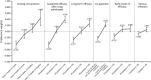
Psoriasis is a chronic autoimmune disease affecting skin which may also manifest in nails and joints. Several biologic treatments have been approved in Japan for psoriasis. Each biologic has a different profile for efficacy and safety, including different dosing regimens and co-payment considerations which may complicate treatment decisions made by patients and physicians during short consultations. Elucidating patient preference is expected to contribute to shared decision-making between patients and physicians to optimize treatment satisfaction and outcomes. However, the number of studies investigating this in Japan is very limited. The study used a discrete choice experiment methodology to elicit patient preferences for hypothetical options in an experimental framework. Participants were asked to choose their preferred treatment option from two hypothetical choices, defined by different levels of six attributes (i.e. early onset of efficacy, long-term efficacy, sustained efficacy after drug withdrawal, dosing convenience, co-payment and risk of serious infection). The survey included 16 treatment choice scenarios and was completed by 395 participants. Across all participants, the attribute regarded as most important was sustained efficacy after drug withdrawal, followed by dosing convenience, co-payment, long-term efficacy, early onset of efficacy and risk of serious infection. The study found that patients prefer treatments which have durable efficacy and lower treatment burden characterized as fewer injection frequency and lower co-payment. These results may be helpful to understand patient preference for biologic treatments used for psoriasis in Japan and contribute to shared decision-making between patients and physicians to improve patient satisfaction and treatment outcomes.
Keywords: Japan; biologics; discrete choice experiment; patient preference; psoriasis.
© 2019 AbbVie GK. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
Y. T. has received honoraria for research from Maruho, LEO Pharma, Eisai, AbbVie, Kyowa Hakko Kirin, Celgene, Meiji‐Seika‐pharma, Taiho and Eli Lilly & Co., and for lecturing from Maruho, Kyowa Hakko Kirin, LEO Pharma, Eli Lilly & Co. and Janssen Pharmaceutical. K. I., J. K. and I. K. are full‐time employees of AbbVie GK and may own AbbVie stock. Keigo Hanada is an employee of CRECON Medical Assessment Inc. that was paid to conduct analyses for this article.
Figures



References
-
- Takahashi H, Nakamura K, Kaneko F, Nakagawa H, Iizuka H, Japanese Society for Psoriasis Research . Analysis of psoriasis patients registered with the Japanese Society for Psoriasis Research from 2002‐2008. J Dermatol 2011; 38(12): 1125–1129. - PubMed
-
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017; 31(2): 205–212. - PubMed
-
- Boehncke W‐H, Schön MP. Psoriasis. Lancet 2015; 386(9997): 983–994. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

