Mania- and anxiety-like behavior and impaired maternal care in female diacylglycerol kinase eta and iota double knockout mice
- PMID: 30985063
- PMCID: PMC6800745
- DOI: 10.1111/gbb.12570
Mania- and anxiety-like behavior and impaired maternal care in female diacylglycerol kinase eta and iota double knockout mice
Abstract
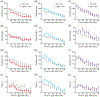
Genome-wide association studies linked diacylglycerol kinase eta and iota to mood disorders, including bipolar disorder and schizophrenia, and both genes are expressed throughout the brain. Here, we generated and behaviorally characterized female mice lacking Dgkh alone, Dgki alone, and double Dgkh/Dgki-knockout (dKO) mice. We found that fewer than 30% of newborn pups raised by dKO females survived to weaning, while over 85% of pups survived to weaning when raised by wild-type (WT) females. Poor survival under the care of dKO mothers was unrelated to pup genotype. Moreover, pups from dKO dams survived when fostered by WT dams, suggesting the poor survival rate of dKO-raised litters was related to impaired maternal care by dKO dams. Nest building was similar between WT and dKO dams; however, some dKO females failed to retrieve any pups in a retrieval assay. Pups raised by dKO dams had smaller or absent milk spots and reduced weight, indicative of impaired nursing. Unlike WT females, postpartum dKO females showed erratic, panicked responses to cage disturbances. Virgin dKO females showed behavioral signs of anxiety and mania, which were not seen in mice lacking either Dgkh or Dgki alone. Our research indicates that combined deletion of Dgkh and Dgki impairs maternal behavior in the early postpartum period, and suggests female dKO mice model symptoms of mania and anxiety.
Keywords: Dgkh; Dgki; anxiety; bipolar disorder; diacylglycerol kinase; female mice; knockout mice; mania; maternal behavior; mouse behavior; postpartum.
© 2019 John Wiley & Sons Ltd and International Behavioural and Neural Genetics Society.
Figures



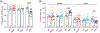

References
-
- Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409(1):1–18. - PubMed
-
- Tu-Sekine B, Raben DM. Regulation and roles of neuronal diacylglycerol kinases: a lipid perspective. Crit Rev Biochem Mol Biol. 2011;46(5):353–364. - PubMed
-
- Kim K, Yang J, Kim E. Diacylglycerol kinases in the regulation of dendritic spines. J Neurochem. 2010;112(3):577–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

