Association of Closed-Loop Brain Stimulation Neurophysiological Features With Seizure Control Among Patients With Focal Epilepsy
- PMID: 30985902
- PMCID: PMC6583077
- DOI: 10.1001/jamaneurol.2019.0658
Association of Closed-Loop Brain Stimulation Neurophysiological Features With Seizure Control Among Patients With Focal Epilepsy
Abstract
Importance: A bidirectional brain-computer interface that performs neurostimulation has been shown to improve seizure control in patients with refractory epilepsy, but the therapeutic mechanism is unknown.
Objective: To investigate whether electrographic effects of responsive neurostimulation (RNS), identified in electrocorticographic (ECOG) recordings from the device, are associated with patient outcomes.
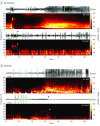
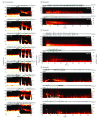
Design, setting, and participants: Retrospective review of ECOG recordings and accompanying clinical meta-data from 11 consecutive patients with focal epilepsy who were implanted with a neurostimulation system between January 28, 2015, and June 6, 2017, with 22 to 112 weeks of follow-up. Recorded ECOG data were obtained from the manufacturer; additional system-generated meta-data, including recording and detection settings, were collected directly from the manufacturer's management system using an in-house, custom-built platform. Electrographic seizure patterns were identified in RNS recordings and evaluated in the time-frequency domain, which was locked to the onset of the seizure pattern.
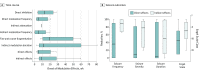
Main outcomes and measures: Patterns of electrophysiological modulation were identified and then classified according to their latency of onset in relation to triggered stimulation events. Seizure control after RNS implantation was assessed by 3 main variables: mean frequency of seizure occurrence, estimated mean severity of seizures, and mean duration of seizures. Overall seizure outcomes were evaluated by the extended Personal Impact of Epilepsy Scale questionnaires, a patient-reported outcome measure of 3 domains (seizure characteristics, medication adverse effects, and quality of life), with a range of possible scores from 0 to 300 in which lower scores indicate worse status, and the Engel scale, which comprises 4 classes (I-IV) in which lower numbers indicate greater improvement.
Results: Electrocorticographic data from 11 patients (8 female; mean [range] age, 35 [19-65] years; mean [range] duration of epilepsy, 19 [5-37] years) were analyzed. Two main categories of electrophysiological signatures of stimulation-induced modulation of the seizure network were discovered: direct and indirect effects. Direct effects included ictal inhibition and early frequency modulation but were not associated with improved clinical outcomes (odds ratio [OR], 0.67; 95% CI, 0.06-7.35; P > .99). Only indirect effects-those occurring remote from triggered stimulation-were associated with improved clinical outcomes (OR, infinity; 95% CI, -infinity to infinity; P = .02). These indirect effects included spontaneous ictal inhibition, frequency modulation, fragmentation, and ictal duration modulation.
Conclusions and relevance: These findings suggest that RNS effectiveness may be explained by long-term, stimulation-induced modulation of seizure network activity rather than by direct effects on each detected seizure.
Conflict of interest statement
Figures


References
-
- Penfield W, Jasper H. Electrocorticography In: Epilepsy and the Functional Anatomy of the Human Brain. Boston, MA: Little Brown; 1954.

