Human Multilineage 3D Spheroids as a Model of Liver Steatosis and Fibrosis
- PMID: 30986904
- PMCID: PMC6480107
- DOI: 10.3390/ijms20071629
Human Multilineage 3D Spheroids as a Model of Liver Steatosis and Fibrosis
Abstract
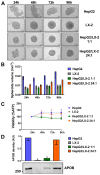
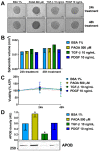
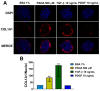
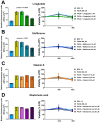
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disorder in western countries. Despite the high prevalence of NAFLD, the underlying biology of the disease progression is not clear, and there are no approved drugs to treat non-alcoholic steatohepatitis (NASH), the most advanced form of the disease. Thus, there is an urgent need for developing advanced in vitro human cellular systems to study disease mechanisms and drug responses. We attempted to create an organoid system genetically predisposed to NAFLD and to induce steatosis and fibrosis in it by adding free fatty acids. We used multilineage 3D spheroids composed by hepatocytes (HepG2) and hepatic stellate cells (LX-2) with a physiological ratio (24:1). HepG2 and LX-2 cells are homozygotes for the PNPLA3 I148M sequence variant, the strongest genetic determinant of NAFLD. We demonstrate that hepatic stellate cells facilitate the compactness of 3D spheroids. Then, we show that the spheroids develop accumulations of fat and collagen upon exposure to free fatty acids. Finally, this accumulation was rescued by incubating spheroids with liraglutide or elafibranor, drugs that are in clinical trials for the treatment of NASH. In conclusion, we have established a simple, easy to handle, in vitro model of genetically induced NAFLD consisting of multilineage 3D spheroids. This tool may be used to understand molecular mechanisms involved in the early stages of fibrogenesis induced by lipid accumulation. Moreover, it may be used to identify new compounds to treat NASH using high-throughput drug screening.
Keywords: NAFLD; NASH; PNPLA3; elafibranor; fatty acids; fibrosis; liraglutide; obeticholic acid; organoids; vitamin E.
Conflict of interest statement
S.R. has been consulting for AstraZeneca, GSK, Celgene Corporation, and Pfizer in the last 5 years and received the research grant from AstraZeneca. D.L. and S.P. are employees at AstraZeneca. All other authors have none to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2016-01527/Vetenskapsrådet
- 20120533/Hjärt-Lungfonden
- SU 2018-04276/the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement
- Excellence Project, 9321-430/Novonordisk Foundation Grant for Excellence in Endocrinology
- DIA 2017-205/Swedish Diabetes Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

