LOXL2-A New Target in Antifibrogenic Therapy?
- PMID: 30986934
- PMCID: PMC6480111
- DOI: 10.3390/ijms20071634
LOXL2-A New Target in Antifibrogenic Therapy?
Abstract
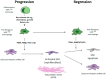
The concept of liver fibrosis and cirrhosis being static and therefore irreversible is outdated. Indeed, both human and animal studies have shown that fibrogenesis is a dynamic and potentially reversible process that can be modulated either by stopping its progression and/or by promoting its resolution. Therefore, the study of the molecular mechanisms involved in the pathogenesis of liver fibrosis is critical for the development of future antifibrotic therapies. The fibrogenesis process, common to all forms of liver injury, is characterized by the increased deposition of extracellular matrix components (EMCs), including collagen, proteoglycans, and glycoproteins (laminin and fibronectin 2). These changes in the composition of the extracellular matrix components alter their interaction with cell adhesion molecules, influencing the modulation of cell functions (growth, migration, and gene expression). Hepatic stellate cells and Kupffer cells (liver macrophages) are the key fibrogenic effectors. The antifibrogenic mechanism starts with the activation of Ly6Chigh macrophages, which can differentiate into macrophages with antifibrogenic action. The research of biochemical changes affecting fibrosis irreversibility has identified lysyl oxidase-like 2 (LOXL2), an enzyme that promotes the network of collagen fibers of the extracellular matrix. LOXL2 inhibition can decrease cell numbers, proliferation, colony formations, and cell growth, and it can induce cell cycle arrest and increase apoptosis. The development of a new humanized IgG4 monoclonal antibody against LOXL2 could open the window of a new antifibrogenic treatment. The current therapeutic target in patients with liver cirrhosis should focus (after the eradication of the causal agent) on the development of new antifibrogenic drugs. The development of these drugs must meet three premises: Patient safety, in non-cirrhotic phases, down-staging or at least stabilization and slowing the progression to cirrhosis must be achieved; whereas in the cirrhotic stage, the objective should be to reduce fibrosis and portal pressure.
Keywords: LOXL2; fibrosis; hepatic stellate cells; portal hypertension; regression cirrhosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

