Apigenin Inhibits IL-31 Cytokine in Human Mast Cell and Mouse Skin Tissues
- PMID: 30987029
- PMCID: PMC6479805
- DOI: 10.3390/molecules24071290
Apigenin Inhibits IL-31 Cytokine in Human Mast Cell and Mouse Skin Tissues
Abstract
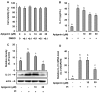
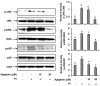
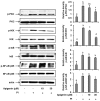
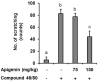
IL-31 is a recently discovered cytokine that is produced not only in T-cells but also in mast cells. It is strongly implicated to play a key role in inflammatory diseases and in the pathogenesis of itch in atopic dermatitis. Apigenin, a flavonoid of plant origin has numerous biological applications. In this study, we showed that apigenin modulates IL-31 mRNA, protein expression, and release in stimulated human mast (HMC-1) by inhibiting the phosphorylation activation of MAPK and NF-κB. To determine whether apigenin has similar effects in vivo, using Compound 48/80, we developed an atopic dermatitis itch model in mice and found an increase in IL-31 expression in the skin. We also revealed that apigenin prevents the infiltration and degranulation of mast cells and suppressed mRNA and protein expression of IL-31 in the skin of mice. These results provide a new suggestion of the potential applicability of apigenin for treatment of various inflammatory diseases and itch mediated by IL-31.
Keywords: IL-31; MAPK; NF-κB; apigenin; atopic dermatitis; mast cells.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures
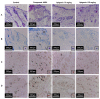
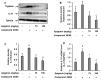
Similar articles
-
Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer.Int J Mol Sci. 2023 Jan 12;24(2):1498. doi: 10.3390/ijms24021498. Int J Mol Sci. 2023. PMID: 36675015 Free PMC article. Review.
-
Sopoongsan inhibits mast cell-mediated anaphylactic reactions and inflammatory cytokine secretion.Int Arch Allergy Immunol. 2006;139(1):31-7. doi: 10.1159/000089520. Epub 2005 Nov 3. Int Arch Allergy Immunol. 2006. PMID: 16272824
-
Huang-Lian-Jie-Du extract ameliorates atopic dermatitis-like skin lesions induced by 2,4-dinitrobenzene in mice via suppression of MAPKs and NF-κB pathways.J Ethnopharmacol. 2020 Mar 1;249:112367. doi: 10.1016/j.jep.2019.112367. Epub 2019 Oct 31. J Ethnopharmacol. 2020. PMID: 31678637
-
Soybean Fermented with Bacillus amyloliquefaciens (Cheonggukjang) Ameliorates Atopic Dermatitis-Like Skin Lesion in Mice by Suppressing Infiltration of Mast Cells and Production of IL-31 Cytokine.J Microbiol Biotechnol. 2019 May 28;29(5):827-837. doi: 10.4014/jmb.1812.12046. J Microbiol Biotechnol. 2019. PMID: 30982315
-
Mouse mast cell cytokine production: rôle in cutaneous inflammatory and immunological responses.Exp Dermatol. 1995 Aug;4(4 Pt 2):240-9. doi: 10.1111/j.1600-0625.1995.tb00252.x. Exp Dermatol. 1995. PMID: 8528596 Review.
Cited by
-
In Vitro Safety and Efficacy Evaluation of a Juniperus communis Callus Culture Extract and Matricaria recutita Processing Waste Extract Combination as a Cosmetic Ingredient.Plants (Basel). 2024 Jan 18;13(2):287. doi: 10.3390/plants13020287. Plants (Basel). 2024. PMID: 38256840 Free PMC article.
-
Apigenin accelerates wound healing in diabetic mice by promoting macrophage M2-type polarization via increasing miR-21 expression.Mol Cell Biochem. 2024 Nov;479(11):3119-3127. doi: 10.1007/s11010-023-04885-y. Epub 2024 Jan 23. Mol Cell Biochem. 2024. PMID: 38261238
-
Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer.Int J Mol Sci. 2023 Jan 12;24(2):1498. doi: 10.3390/ijms24021498. Int J Mol Sci. 2023. PMID: 36675015 Free PMC article. Review.
-
Analgesic and anti-inflammatory effects of galangin: a potential pathway to inhibit transient receptor potential vanilloid 1 receptor activation.Korean J Pain. 2024 Apr 1;37(2):151-163. doi: 10.3344/kjp.23363. Korean J Pain. 2024. PMID: 38557656 Free PMC article.
-
New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression.J Clin Med. 2021 Jun 5;10(11):2506. doi: 10.3390/jcm10112506. J Clin Med. 2021. PMID: 34198894 Free PMC article. Review.
References
-
- Niyonsaba F., Ushio H., Hara M., Yokoi H., Tominaga M., Takamori K., Kajiwara N., Saito H., Nagaoka I., Ogawa H., et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010;184:3526–3534. doi: 10.4049/jimmunol.0900712. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

