Discovery of Novel Triazole-Containing Pyrazole Ester Derivatives as Potential Antibacterial Agents
- PMID: 30987179
- PMCID: PMC6480153
- DOI: 10.3390/molecules24071311
Discovery of Novel Triazole-Containing Pyrazole Ester Derivatives as Potential Antibacterial Agents
Abstract
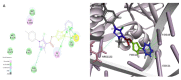
To develop new antibacterial agents, a series of novel triazole-containing pyrazole ester derivatives were designed and synthesized and their biological activities were evaluated as potential topoisomerase II inhibitors. Compound 4d exhibited the most potent antibacterial activity with Minimum inhibitory concentration (MIC) alues of 4 µg/mL, 2 µg/mL, 4 µg/mL, and 0.5 µg/mL against Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella gallinarum, respectively. The in vivo enzyme inhibition assay 4d displayed the most potent topoisomerase II (IC50 = 13.5 µg/mL) and topoisomerase IV (IC50 = 24.2 µg/mL) inhibitory activity. Molecular docking was performed to position compound 4d into the topoisomerase II active site to determine the probable binding conformation. In summary, compound 4d may serve as potential topoisomerase II inhibitor.
Keywords: antibacterial; ester; pyrazole; topoisomerase II inhibitor; triazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Esteves-Souza A., Rodrigues-Santos C.E., Del Cistia Cde N., Silva D.R., Sant’anna C.M., Echevarria A. Solvent-free synthesis, DNA-topoisomerase II activity and molecular docking study of new asymmetrically N,N’-substituted ureas. Molecules. 2012;17:12882–12894. doi: 10.3390/molecules171112882. - DOI - PMC - PubMed
-
- Gellerman G. Recent Developments in the Synthesis and Applications of Anticancer Amonafide Derivatives. A Mini Review. Lett. Drug Des. Discov. 2016;13:47–63. doi: 10.2174/1570180812666150529205049. - DOI
-
- Mcgarry D.H., Cooper I.R., Walker R., Warrilow C.E., Pichowicz M., Ratcliffe A.J., Salisbury A.M., Savage V.J., Moyo E., Maclean J. Design, synthesis and antibacterial properties of pyrimido[4,5-b]indol-8-amine inhibitors of DNA gyrase. Bioorg. Med. Chem. Lett. 2018;28:2998–3003. doi: 10.1016/j.bmcl.2018.05.049. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

