Metalloproteins in the Biology of Heterocysts
- PMID: 30987221
- PMCID: PMC6616624
- DOI: 10.3390/life9020032
Metalloproteins in the Biology of Heterocysts
Abstract
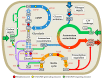
Cyanobacteria are photoautotrophic microorganisms present in almost all ecologically niches on Earth. They exist as single-cell or filamentous forms and the latter often contain specialized cells for N₂ fixation known as heterocysts. Heterocysts arise from photosynthetic active vegetative cells by multiple morphological and physiological rearrangements including the absence of O₂ evolution and CO₂ fixation. The key function of this cell type is carried out by the metalloprotein complex known as nitrogenase. Additionally, many other important processes in heterocysts also depend on metalloproteins. This leads to a high metal demand exceeding the one of other bacteria in content and concentration during heterocyst development and in mature heterocysts. This review provides an overview on the current knowledge of the transition metals and metalloproteins required by heterocysts in heterocyst-forming cyanobacteria. It discusses the molecular, physiological, and physicochemical properties of metalloproteins involved in N₂ fixation, H₂ metabolism, electron transport chains, oxidative stress management, storage, energy metabolism, and metabolic networks in the diazotrophic filament. This provides a detailed and comprehensive picture on the heterocyst demands for Fe, Cu, Mo, Ni, Mn, V, and Zn as cofactors for metalloproteins and highlights the importance of such metalloproteins for the biology of cyanobacterial heterocysts.
Keywords: Nostocales; bioenergetics; cyanobacteria; electron transport chains; heterocysts; metabolism; metalloenzymes; metalloproteins; metals; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- International Union of Biochemistry. Nomenclature C., Webb E.C. Enzyme Nomenclature, 1992: Recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology on the Nomenclature and Classification of Enzymes. Published for the International Union of Biochemistry and Molecular Biology by Academic Press, Inc.; San Diego, CA, USA: 1992.
-
- Da Silva J.F., Williams R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. 2nd ed. Oxford University Press; Oxford, UK: 2001.
-
- Bertini I., Sigel A., Sigel H. Handbook on Metalloproteins. Marcel Dekker; New York, NY, USA: 2001.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

