Small-Molecule Host-Defense Peptide Mimetic Antibacterial and Antifungal Agents Activate Human and Mouse Mast Cells via Mas-Related GPCRs
- PMID: 30987258
- PMCID: PMC6523814
- DOI: 10.3390/cells8040311
Small-Molecule Host-Defense Peptide Mimetic Antibacterial and Antifungal Agents Activate Human and Mouse Mast Cells via Mas-Related GPCRs
Abstract
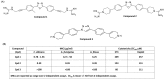
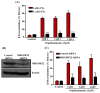
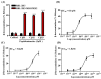
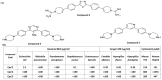
Host-defense peptides (HDPs) have an important therapeutic potential against microbial infections but their metabolic instability and cellular cytotoxicity have limited their utility. To overcome these limitations, we utilized five small-molecule, nonpeptide HDP mimetics (smHDPMs) and tested their effects on cytotoxicity, antimicrobial activity, and mast cell (MC) degranulation. None of the smHDPMs displayed cytotoxicity against mouse 3T3 fibroblasts or human transformed liver HepG2 cells. However, one compound had both antifungal and antibacterial activity. Surprisingly, all five compounds induced degranulation in a human MC line, LAD2, and this response was substantially reduced in Mas-related G protein-coupled receptor (GPCR)-X2 (MRGPRX2)-silenced cells. Furthermore, all five compounds induced degranulation in RBL-2H3 cells expressing MRGPRX2 but this response was abolished in cells expressing naturally occurring loss-of-function missense variants G165E (rs141744602) and D184H (rs372988289). Mrgprb2 is the likely mouse ortholog of human MRGPRX2, which is expressed in connective tissue MCs (CTMCs) such as cutaneous and peritoneal MCs (PMCs). All five smHDPMs induced degranulation in wild-type PMCs but not in cells derived from Mrgprb2⁻/⁻ mice. These findings suggest that smHDPMs could serve as novel targets for the treatment of drug-resistant fungal and bacterial infections because of their ability to harness CTMCs' host defense functions.
Keywords: Host-defense peptide mimetics; MRGPRX2; Mast cells; Mrgprb2.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


References
-
- Shiota N., Nishikori Y., Kakizoe E., Shimoura K., Niibayashi T., Shimbori C., Tanaka T., Okunishi H. Pathophysiological role of skin mast cells in wound healing after scald injury: Study with mast cell-deficient W/W(V) mice. Int. Arch. Allergy Immunol. 2010;151:80–88. doi: 10.1159/000232573. - DOI - PubMed
-
- Groschwitz K.R., Ahrens R., Osterfeld H., Gurish M.F., Han X., Åbrink M., Finkelman F.D., Pejler G., Hogan S.P. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl. Acad. Sci. USA. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. - DOI - PMC - PubMed
-
- Arifuzzaman M., Mobley Y.R., Choi H.W., Bist P., Salinas C.A., Brown Z.D., Chen S.L., Staats H.F., Abraham S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019;5:eaav0216. doi: 10.1126/sciadv.aav0216. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

