MCR Scaffolds Get Hotter with 18F-Labeling
- PMID: 30987302
- PMCID: PMC6480256
- DOI: 10.3390/molecules24071327
MCR Scaffolds Get Hotter with 18F-Labeling
Abstract
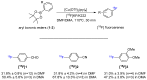
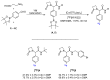
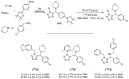
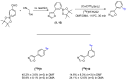
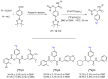
Imaging techniques, such as positron emission tomography (PET), represent great progress in the clinical development of drugs and diagnostics. However, the efficient and timely synthesis of appropriately labeled compounds is a largely unsolved problem. Numerous small drug-like molecules with high structural diversity can be synthesized via convergent multicomponent reactions (MCRs). The combination of PET labeling with MCR synthesis of biologically active compounds can greatly simplify radioanalytical and imaging-based analysis. In a proof-of-concept study, we optimized robust on-site radiolabeling conditions that were subsequently applied to several structurally different drug-like MCR scaffolds (e.g., arenes, β-lactam, tetrazole, and oxazole). These labeled scaffolds were synthesized via pinacol-derived aryl boronic esters (arylBPin) by copper-mediated oxidative 18F-fluorination with radiochemical conversions (RCCs) from 15% to 76%.
Keywords: boronic pinacol esters; fluor-18; multicomponent reactions; positron emission tomography; radiochemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

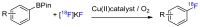

References
-
- Patel S., Schmidt K., Hesterman J., Hoppin J. Advancing Drug Discovery and Development Using Molecular Imaging (ADDMI): An Interest Group of the World Molecular Imaging Society and an Inaugural Session on Positron Emission Tomography (PET) Mol. Imaging Biol. 2017;19:348–356. doi: 10.1007/s11307-017-1085-7. - DOI - PubMed
-
- Slobbe P., Ruijter E., Orru R.V.A. Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm. 2012;3:1189–1218. doi: 10.1039/C2MD20089A. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

