Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates
- PMID: 30988006
- PMCID: PMC6597842
- DOI: 10.1074/jbc.RA119.007528
Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates
Abstract
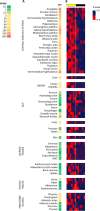
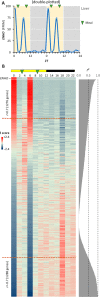
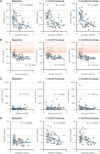
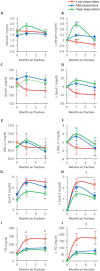
Mouse studies linking adropin, a peptide hormone encoded by the energy homeostasis-associated (ENHO) gene, to biological clocks and to glucose and lipid metabolism suggest a potential therapeutic target for managing diseases of metabolism. However, adropin's roles in human metabolism are unclear. In silico expression profiling in a nonhuman primate diurnal transcriptome atlas (GSE98965) revealed a dynamic and diurnal pattern of ENHO expression. ENHO expression is abundant in brain, including ventromedial and lateral hypothalamic nuclei regulating appetite and autonomic function. Lower ENHO expression is present in liver, lung, kidney, ileum, and some endocrine glands. Hepatic ENHO expression associates with genes involved in glucose and lipid metabolism. Unsupervised hierarchical clustering identified 426 genes co-regulated with ENHO in liver, ileum, kidney medulla, and lung. Gene Ontology analysis of this cluster revealed enrichment for epigenetic silencing by histone H3K27 trimethylation and biological processes related to neural function. Dietary intervention experiments with 59 adult male rhesus macaques indicated low plasma adropin concentrations were positively correlated with fasting glucose, plasma leptin, and apolipoprotein C3 (APOC3) concentrations. During consumption of a high-sugar (fructose) diet, which induced 10% weight gain, animals with low adropin had larger increases of plasma leptin and more severe hyperglycemia. Declining adropin concentrations were correlated with increases of plasma APOC3 and triglycerides. In summary, peripheral ENHO expression associates with pathways related to epigenetic and neural functions, and carbohydrate and lipid metabolism, suggesting co-regulation in nonhuman primates. Low circulating adropin predicts increased weight gain and metabolic dysregulation during consumption of a high-sugar diet.
Keywords: apolipoprotein; circadian; dyslipidemia; epigenetics; glucose metabolism; insulin resistance; leptin; nutrition; obesity; type 2 diabetes.
© 2019 Butler et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Comment in
-
Setting fire to fat.J Biol Chem. 2019 Jun 21;294(25):9720-9721. doi: 10.1074/jbc.H119.009488. J Biol Chem. 2019. PMID: 31227622 Free PMC article.
References
-
- Kumar K. G., Trevaskis J. L., Lam D. D., Sutton G. M., Koza R. A., Chouljenko V. N., Kousoulas K. G., Rogers P. M., Kesterson R. A., Thearle M., Ferrante A. W. Jr., Mynatt R. L., Burris T. P., Dong J. Z., Halem H. A., et al. (2008) Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 8, 468–481 10.1016/j.cmet.2008.10.011 - DOI - PMC - PubMed
-
- Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C. A., et al. (2015) Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 10.1126/science.1260419 - DOI - PubMed
-
- Sato K., Yamashita T., Shirai R., Shibata K., Okano T., Yamaguchi M., Mori Y., Hirano T., and Watanabe T. (2018) Adropin contributes to anti-atherosclerosis by suppressing monocyte-endothelial cell adhesion and smooth muscle cell proliferation. Int. J. Mol. Sci. 19, E1293 10.3390/ijms19051293 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

