Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity
- PMID: 30988051
- PMCID: PMC6503947
- DOI: 10.1242/jeb.190058
Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity
Abstract
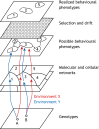
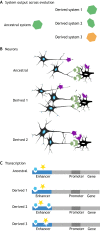
We propose that insights from the field of evolutionary developmental biology (or 'evo-devo') provide a framework for an integrated understanding of the origins of behavioural diversity and its underlying mechanisms. Towards that goal, in this Commentary, we frame key questions in behavioural evolution in terms of molecular, cellular and network-level properties with a focus on the nervous system. In this way, we highlight how mechanistic properties central to evo-devo analyses - such as weak linkage, versatility, exploratory mechanisms, criticality, degeneracy, redundancy and modularity - affect neural circuit function and hence the range of behavioural variation that can be filtered by selection. We outline why comparative studies of molecular and neural systems throughout ontogeny will provide novel insights into diversity in neural circuits and behaviour.
Keywords: Canalization; Modularity; Neural circuits; Neuroethology; Plasticity; Robustness.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Conservation Evo-Devo: Preserving Biodiversity by Understanding Its Origins.Trends Ecol Evol. 2017 Oct;32(10):746-759. doi: 10.1016/j.tree.2017.07.002. Epub 2017 Aug 7. Trends Ecol Evol. 2017. PMID: 28797609
-
Demystifying phenotypes: The comparative genomics of evo-devo.Fly (Austin). 2010 Jan-Mar;4(1):18-20. doi: 10.4161/fly.4.1.10509. Epub 2010 Jan 2. Fly (Austin). 2010. PMID: 19955851
-
Where is, in 2017, the evo in evo-devo (evolutionary developmental biology)?J Exp Zool B Mol Dev Evol. 2018 Jan;330(1):15-22. doi: 10.1002/jez.b.22791. Epub 2018 Feb 2. J Exp Zool B Mol Dev Evol. 2018. PMID: 29393575
-
A way forward with eco evo devo: an extended theory of resource polymorphism with postglacial fishes as model systems.Biol Rev Camb Philos Soc. 2019 Oct;94(5):1786-1808. doi: 10.1111/brv.12534. Epub 2019 Jun 19. Biol Rev Camb Philos Soc. 2019. PMID: 31215138 Free PMC article. Review.
-
Evo-devo and the evolution of social behavior.Trends Genet. 2007 Jul;23(7):334-41. doi: 10.1016/j.tig.2007.05.001. Epub 2007 May 16. Trends Genet. 2007. PMID: 17509723 Review.
Cited by
-
Back to the basics? Transcriptomics offers integrative insights into the role of space, time and the environment for gene expression and behaviour.Biol Lett. 2021 Sep;17(9):20210293. doi: 10.1098/rsbl.2021.0293. Epub 2021 Sep 15. Biol Lett. 2021. PMID: 34520681 Free PMC article.
-
Evolution of Reproductive Behavior.Genetics. 2020 Jan;214(1):49-73. doi: 10.1534/genetics.119.302263. Genetics. 2020. PMID: 31907301 Free PMC article. Review.
-
Neuroanatomical and neurophysiological mechanisms of acoustic and weakly electric signaling in synodontid catfish.J Comp Neurol. 2020 Oct 15;528(15):2602-2619. doi: 10.1002/cne.24920. Epub 2020 Apr 21. J Comp Neurol. 2020. PMID: 32266714 Free PMC article.
-
The power and limitations of gene expression pathway analyses toward predicting population response to environmental stressors.Evol Appl. 2020 Mar 3;13(6):1166-1182. doi: 10.1111/eva.12935. eCollection 2020 Jul. Evol Appl. 2020. PMID: 32684953 Free PMC article.
-
Inspiring song: The role of respiratory circuitry in the evolution of vertebrate vocal behavior.Dev Neurobiol. 2020 Jan;80(1-2):31-41. doi: 10.1002/dneu.22752. Epub 2020 May 19. Dev Neurobiol. 2020. PMID: 32329162 Free PMC article. Review.
References
-
- Anderson M. L. (2007). The massive redeployment hypothesis and the functional topography of the brain. Philos. Psychol. 20, 143-174. 10.1080/09515080701197163 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

