T cell anergy in perinatal mice is promoted by T reg cells and prevented by IL-33
- PMID: 30988052
- PMCID: PMC6547863
- DOI: 10.1084/jem.20182002
T cell anergy in perinatal mice is promoted by T reg cells and prevented by IL-33
Abstract
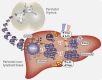
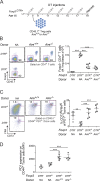
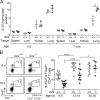
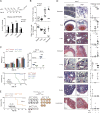
Perinatal T cells broadly access nonlymphoid tissues, where they are exposed to sessile tissue antigens. To probe the outcome of such encounters, we examined the defective elimination of self-reactive clones in Aire-deficient mice. Nonlymphoid tissues were sequentially seeded by distinct waves of CD4+ T cells. Early arrivers were mostly Foxp3+ regulatory T (T reg) cells and metabolically active, highly proliferative conventional T cells (T conv cells). T conv cells had unusually high expression of PD-1 and the IL-33 receptor ST2. As T conv cells accumulated in the tissue, they gradually lost expression of ST2, ceased to proliferate, and acquired an anergic phenotype. The transition from effector to anergic state was substantially faster in ST2-deficient perinates, whereas it was abrogated in IL-33-treated mice. A similar dampening of anergy occurred after depletion of perinatal T reg cells. Attenuation of anergy through PD-1 blockade or IL-33 administration promoted the immediate breakdown of tolerance and onset of multiorgan autoimmunity. Hence, regulating IL-33 availability may be critical in maintaining T cell anergy.
© 2019 Tuncel et al.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

