Detection of NRG1 Gene Fusions in Solid Tumors
- PMID: 30988082
- PMCID: PMC7470623
- DOI: 10.1158/1078-0432.CCR-19-0160
Detection of NRG1 Gene Fusions in Solid Tumors
Abstract
Purpose: NRG1 gene fusions are rare but potentially actionable oncogenic drivers that are present in some solid tumors. Details regarding the incidence of these gene rearrangements are lacking. Here, we assessed the incidence of NRG1 fusions across multiple tumor types and described fusion partners.
Experimental design: Tumor specimens submitted for molecular profiling at a Clinical Laboratory Improvement Amendments (CLIA)-certified genomics laboratory and that underwent fusion testing by anchored multiplex PCR for targeted RNA sequencing were retrospectively identified. The overall and tumor-specific incidence was noted, as was the specific fusion partner.
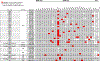
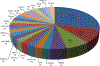
Results: Out of 21,858 tumor specimens profiled from September 2015 to December 2018, 41 cases (0.2%) harbored an NRG1 fusion. Multiple fusion partners were identified. Fusion events were seen across tumor types. The greatest incidence was in non-small cell lung cancer (NSCLC, 25), though this represented only 0.3% of NSCLC cases tested. Other tumor types harboring an NRG1 fusion included gallbladder cancer, renal cell carcinoma, bladder cancer, ovarian cancer, pancreatic cancer, breast cancer, neuroendocrine tumor, sarcoma, and colorectal cancer.
Conclusions: NRG1 fusions can be detected at a low incidence across multiple tumor types with significant heterogeneity in fusion partner.See related commentary by Dimou and Camidge, p. 4865.
©2019 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Statement: S. Jonna reports no competing interests. R.A. Feldman, J. Swensen, Z. Gatalica and W.M. Korn are employees of Caris Life Sciences. W.M. Korn reports consulting/advisory role from Merrimack and Merck Sharp & Dohme, stock/ownership interests with OncoCyte, has received honoraria from Genentech/Roche and institution-associated funding from Merrimack. H. Borghaei reports consulting/advisory role from Bristol-Myers Squibb, Lilly, Celgene, Genentech, Pfizer, Boehringer Ingelheim, EMD Serono, Trovagene, Novartis, Merck, AstraZeneca, Genmab, Regeneron, Contargia AB, BioNTech AG, Abbvie, received honoraria from Bristol-Myers Squibb, Celgene, Axiom Biotechnologies and institution-associated research funding from Millennium, Merck, Celgene, Bristol-Myers Squibb and Lilly. P.C. Ma reports association with the speakers’ bureau for Merck, Takeda, Bristol-Myers Squibb, stock/ownership from Cymeta Biopharmaceutical and institution-associated research funding from Pfizer, MedImmune, AstraZeneca, Bristol-Myers Squibb, Loxo, Xcovery, Abbvie, EpicentRx, Tesaro, Boehringer Ingelheim, OncoMed, CBT Pharmaceuticals, Merck and Cymeta Biopharmaceutical. J. Nieva reports consulting/advisory role with AstraZeneca, Genentech/Roche and Western Oncolytics, stock/ownership in Epic Sciences and research funding from Merck. A. Spira reports institution-associated research funding from Roche, AstraZeneca, Boehringer Ingelheim, Astellas Pharma, MedImmune, Novartis, Newlink Genetics, Incyte, Abbvie, Ignyta, LAM Therapeutics, Trovagene, Takeda, Macrogenics and CytomX Therapeutics. A. Vanderwalde reports consulting/advisory role with Bristol-Myers Squibb, AstraZeneca, Genentech and Caris Life Sciences, has received honoraria from AstraZeneca and institution-associated research funding from Amgen, Merck, Genentech/Roche, Millennium, AstraZeneca, Polynoma, Lilly, Bristol-Myers Squibb and Amgen. A. Wozniak reports consulting/advisory role with Boehringer Ingelheim, AstraZeneca, ARIAD, Coherus Biosciences, Array BioPharma, Hospira, Quintiles, BeyondSpring Pharmaceuticals, WCCT Global, Epic, Huron Consulting, HUYA Bioscience International and Takeda and has received research funding from Boehringer Ingelheim. E.S. Kim reports consulting/advisory role with Lilly, AstraZeneca, Boehringer Ingelheim, Pfizer, Merck and Takeda, has received honoraria from Lilly, AstraZeneca, Boehringer Ingelheim, Pfizer, Merck and Takeda and research funding from Boehringer Ingelheim, Lilly, Merck, Ignyta and Genentech/Roche. S.V. Liu reports consulting/advisory role with Apollomics, Boehringer-Ingelheim, G1 Therapeutics, Genentech/Roche, Ignyta, Guardant 360, Inivata, Janssen, Pfizer, Lilly, Merck, Taiho Pharmaceutical, Bristol-Myers Squibb, AstraZeneca, Takeda, HERON and Regeneron and has received research funding from Genentech/Roche, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer, OncoMed, Merck, Lycera, AstraZeneca, Ignyta, Molecular Partners, Blueprint Medicines, Lilly and Rain Therapeutics.
Figures

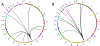

Comment in
-
Detection of NRG1 Fusions in Solid Tumors: Rare Gold?Clin Cancer Res. 2019 Aug 15;25(16):4865-4867. doi: 10.1158/1078-0432.CCR-19-1219. Epub 2019 Jun 11. Clin Cancer Res. 2019. PMID: 31186315
References
-
- Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. - PubMed
-
- Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. - PubMed
-
- Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–16. - PubMed
-
- Mayekar MK, Bivona TG. Current Landscape of Targeted Therapy in Lung Cancer. Clin Pharmacol Ther. 2017;102:757–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

