Trauma Induces Emergency Hematopoiesis through IL-1/MyD88-Dependent Production of G-CSF
- PMID: 30988118
- PMCID: PMC6504560
- DOI: 10.4049/jimmunol.1801456
Trauma Induces Emergency Hematopoiesis through IL-1/MyD88-Dependent Production of G-CSF
Abstract
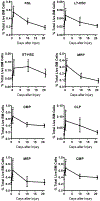
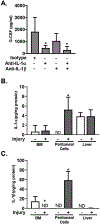
The inflammatory response to infection or injury dramatically increases the hematopoietic demand on the bone marrow to replace effector leukocytes consumed in the inflammatory response. In the setting of infection, pathogen-associated molecular patterns induce emergency hematopoiesis, activating hematopoietic stem and progenitor cells to proliferate and produce progeny for accelerated myelopoiesis. Sterile tissue injury due to trauma also increases leukocyte demand; however, the effect of sterile tissue injury on hematopoiesis is not well described. We find that tissue injury alone induces emergency hematopoiesis in mice subjected to polytrauma. This process is driven by IL-1/MyD88-dependent production of G-CSF. G-CSF induces the expansion of hematopoietic progenitors, including hematopoietic stem cells and multipotent progenitors, and increases the frequency of myeloid-skewed progenitors. To our knowledge, these data provide the first comprehensive description of injury-induced emergency hematopoiesis and identify an IL-1/MyD88/G-CSF-dependent pathway as the key regulator of emergency hematopoiesis after injury.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures






References
-
- Cullen SM, Mayle A, Rossi L, and Goodell MA. 2014. Hematopoietic stem cell development: an epigenetic journey. Current topics in developmental biology 107: 39–75. - PubMed
-
- Manz MG, and Boettcher S. 2014. Emergency granulopoiesis. Nature reviews. Immunology 14: 302–314. - PubMed
-
- Takizawa H, Boettcher S, and Manz MG. 2012. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood 119: 2991–3002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

