Comparable Efficacy and Better Safety of Double β-Lactam Combination Therapy versus β‑Lactam plus Aminoglycoside in Gram-Negative Bacteria in Randomized, Controlled Trials
- PMID: 30988147
- PMCID: PMC6591590
- DOI: 10.1128/AAC.00425-19
Comparable Efficacy and Better Safety of Double β-Lactam Combination Therapy versus β‑Lactam plus Aminoglycoside in Gram-Negative Bacteria in Randomized, Controlled Trials
Abstract
There is a great need for efficacious therapies against Gram-negative bacteria. Double β-lactam combination(s) (DBL) are relatively safe, and preclinical data are promising; however, their clinical role has not been well defined. We conducted a metaanalysis of the clinical and microbiological efficacy of DBL compared to β-lactam plus aminoglycoside combinations (BLAG). PubMed, Embase, ISI Web of Knowledge, and Cochrane Controlled Trials Register database were searched through July 2018. We included randomized controlled clinical trials that compared DBL with BLAG combinations. Clinical response was used as the primary outcome and microbiological response in Gram-negative bacteria as the secondary outcome; sensitivity analyses were performed for Pseudomonas aeruginosa, Klebsiella spp., and Escherichia coli Heterogeneity and risk of bias were assessed. Safety results were classified by systems and organs. Thirteen studies evaluated 2,771 cases for clinical response and 665 cases for microbiological response in various Gram-negative species. DBL achieved slightly, but not significantly, better clinical response (risk ratio, 1.05; 95% confidence interval [CI], 0.99 to 1.11) and microbiological response in Gram-negatives (risk ratio, 1.11; 95% CI, 0.99 to 1.25) compared with BLAG. Sensitivity analyses by pathogen showed the same trend. No significant heterogeneity across studies was found. DBL was significantly safer than BLAG regarding renal toxicity (6.6% versus 8.8%, P = 0.0338) and ototoxicity (0.7 versus 3.1%, P = 0.0137). Other adverse events were largely comparable. Overall, empirically designed DBL showed comparable clinical and microbiological responses across different Gram-negative species, and were significantly safer than BLAG. Therefore, DBL should be rationally optimized via the latest translational approaches, leveraging mechanistic insights and newer β-lactams for future evaluation in clinical trials.
Keywords: Gram-negative bacteria; beta-lactamase inhibitor; combination therapy; double beta-lactam; meta-analysis; randomized controlled clinical trial.
Copyright © 2019 American Society for Microbiology.
Figures
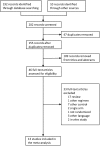
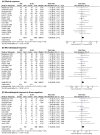
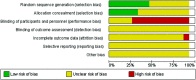
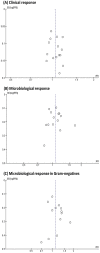
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

