Preoperative chemotherapy and radiotherapy concomitant to cetuximab in resectable stage IIIB NSCLC: a multicentre phase 2 trial (SAKK 16/08)
- PMID: 30988393
- PMCID: PMC6734655
- DOI: 10.1038/s41416-019-0447-0
Preoperative chemotherapy and radiotherapy concomitant to cetuximab in resectable stage IIIB NSCLC: a multicentre phase 2 trial (SAKK 16/08)
Abstract
Background: Neoadjuvant chemotherapy (CT) followed by radiotherapy (RT) and surgery showed a median survival of 28.7 months in resectable stage IIIB non-small-cell lung cancer (NSCLC) patients (pts). Here, we evaluate the impact of concomitant cetuximab to the same neoadjuvant chemo-radiotherapy (CRT) in selected patients (pts) with NSCLC, stage IIIB.
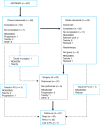
Methods: Resectable stage IIIB NSCLC received three cycles of CT (cisplatin 100 mg/m2 and docetaxel 85 mg/m2 d1, q3w) followed by RT (44 Gy in 22 fractions) with concomitant cetuximab (250 mg/m2, q1w) and subsequent surgery. The primary endpoint was 1-year progression-free survival (PFS).
Results: Sixty-nine pts were included in the trial. Fifty-seven (83%) pts underwent surgery, with complete resection (R0) in 42 (74%) and postoperative 30 day mortality of 3.5%. Responses were: 57% after CT-cetuximab and 64% after CRT-cetuximab. One-year PFS was 50%. Median PFS was 12.0 months (95% CI: 9.0-15.6), median OS was 21.3 months, with a 2- and 3-yr survival of 41% and 30%, respectively.
Conclusions: This is one of the largest prospective phase 2 trial to investigate the role of induction CRT and surgery in resectable stage IIIB disease, and the first adding cetuximab to the neoadjuvant strategy. This trial treatment is feasible with promising response and OS rates, supporting an aggressive approach in selected pts.
Conflict of interest statement
A.C.F. declares receipt of honoraria or consultation fees from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, F. Hoffmann-La Roche, Merck Sharp and Dohme, Novartis, Pfizer and Takeda, as well as honorarium for talks in a company’s organised public event from F. Hoffmann-La Roche and Merck Sharp and Dohme. J.Y.P. received research funding from Hoffmann-La Roche. H.G. declares to have received travel support from Bristol-Myers Squibb. A.X. declares no conflict of interest. H.B. declares receipt of honoraria from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Merck Sharp and Dohme, Pfizer, AstraZeneca and Takeda. N.M. declares receipt of honoraria or consultation fees from Amgen, AstraZeneca, Bristol-Myers Squibb, F. Hoffmann-La Roche, Merck Sharp and Dohme, Merck Serono, Novartis, Pharma Mar, as well as honorarium for talks in a company’s organised public event from Bristol-Myers Squibb and Pharma Mar. O.M. declares no conflict of interest. N.S. declares no conflict of interest. M.F. declares receipt of honoraria or consultation fees from Bristol-Myers Squibb, F. Hoffmann-La Roche, Merck Sharp and Dohme, Astra Zeneca, Boeringer Ingelheim, Pfizer, Takeda. W.W. declares to have received honoraria or consultation fees from Astra Zeneca for Advisory Board and lecture, from Convidien teaching Grant & lecture. R.C. declares receipt of honoraria or consultation fees from Amgen, Astra Zeneca, Bristol-Myers Squibb, Merck Sharp and Dohme, Novartis, Roche, Pfizer, Janssen, Astellas, Bayer, Debiopharm. P.G. declares no conflict of interest. L.B. declares receipt of honoraria or consultation fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Merck Sharp and Dohme, Pfizer and Takeda, as well as honoraria for talks in a company’s organised public event from F. Hoffmann-La Roche and Astra Zeneca. M.P. declares Amgen, Astra Zeneca, BMS, Boehringer, Ingelheim, Lilly, Novartis, Merck, MSD, Roche, Takeda. D.B. declares receipt of honoraria or consultations fees from Amgen, AstraZeneca, Bristol-Myers Squibb, F-Hoffmann-La Roche, Merk Serono, Merck Sharp and Dohme, Novartis, Pfizer, Takeda, Vifor. S.P. declares to have received education grants, provided consultation, attended advisory boards and/or provided lectures for the following organisations, from whom I have received honoraria: Receipt of honoraria or consultation fees: Abbvie, Amgen, AstraZeneca, Bayer, Biocartis, Boehringer-Ingelheim, Bristol-Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, F. Hoffmann-La Roche, Foundation Medicine, Illumina, Janssen, Merck Sharp and Dohme, Merck Serono, Merrimack, Novartis, Pharma Mar, Pfizer, Regeneron, Sanofi, Seattle Genetics and Takeda. Talk in a company’s organised public event: AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Illumina, Merck Sharp and Dohme, Novartis, Pfizer, Sanofi, Takeda. Receipt of grants/research supports: (Sub)investigator in trials (institutional financial support for clinical trials) sponsored by Amgen, AstraZeneca, Biodesix, Boehringer-Ingelheim, Bristol-Myers Squibb, Clovis, F. Hoffmann-La Roche, Illumina, Merck Sharp and Dohme, Merck Serono, Novartis and Pfizer.
Figures


References
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. - DOI - PubMed
-
- Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, 3rd, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J. Clin. Oncol. 1995;13:1880–1892. doi: 10.1200/JCO.1995.13.8.1880. - DOI - PubMed
-
- Stupp R, Mayer M, Kann R, Weder W, Zouhair A, Betticher DC, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol. 2009;10:785–793. doi: 10.1016/S1470-2045(09)70172-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

