Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis
- PMID: 30988423
- PMCID: PMC6556210
- DOI: 10.1038/s41556-019-0309-2
Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis
Abstract
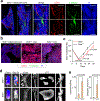
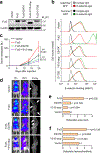
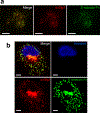
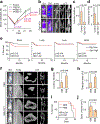
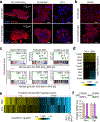
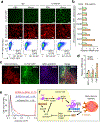
How disseminated tumour cells engage specific stromal components in distant organs for survival and outgrowth is a critical but poorly understood step of the metastatic cascade. Previous studies have demonstrated the importance of the epithelial-mesenchymal transition in promoting the cancer stem cell properties needed for metastasis initiation, whereas the reverse process of mesenchymal-epithelial transition is required for metastatic outgrowth. Here we report that this paradoxical requirement for the simultaneous induction of both mesenchymal-epithelial transition and cancer stem cell traits in disseminated tumour cells is provided by bone vascular niche E-selectin, whose direct binding to cancer cells promotes bone metastasis by inducing mesenchymal-epithelial transition and activating Wnt signalling. E-selectin binding activity mediated by the α1-3 fucosyltransferases Fut3/Fut6 and Glg1 are instrumental to the formation of bone metastasis. These findings provide unique insights into the functional role of E-selectin as a component of the vascular niche critical for metastatic colonization in bone.
Conflict of interest statement
Competing Financial Interests
No other authors declare potential conflicts of interest.
Figures


Comment in
-
E-selectin fills two needs for metastasis.Nat Rev Cancer. 2019 Jun;19(6):301. doi: 10.1038/s41568-019-0151-7. Nat Rev Cancer. 2019. PMID: 31065050 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

