Outcome of Descemet membrane endothelial keratoplasty for graft failure after Descemet stripping automated endothelial keratoplasty
- PMID: 30988597
- PMCID: PMC6438261
- DOI: 10.2147/OPTH.S194185
Outcome of Descemet membrane endothelial keratoplasty for graft failure after Descemet stripping automated endothelial keratoplasty
Abstract
Purpose: To investigate the efficacy and safety of Descemet membrane endothelial keratoplasty (DMEK) for corneal decompensation following primary Descemet stripping automated endothelial keratoplasty (DSAEK).
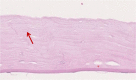
Methods: This was a retrospective case series of 15 patients that underwent DMEK surgery for corneal decompensation after failed DSAEK. Main outcome parameter was corrected distance visual acuity (CDVA) after DMEK and DSAEK. Secondary outcome measures included central corneal thickness (CCT), endothelial cell density (ECD), rebubbling rate, and primary graft failure after DMEK. Explanted DSAEK grafts were evaluated by light microscopy.
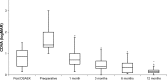
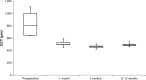
Results: The mean (±SD) time period between DSAEK and DMEK surgery was 15±8 months (range, 6-31 months). Preoperative CDVA was 1.72±0.62 (logMAR). After DMEK, CDVA improved significantly to 0.78±0.48 at 1 month and to 0.23±0.24 after 12 months (P=0.022). Visual acuity data after DMEK were significantly better compared to preoperative values. The average CCT after DMEK decreased significantly from 869±210 µm (preoperative) to 505±45 µm (1 month postoperative) (P<0.001) and remained stable over 12 months. The ECD decreased from 2,589±209/mm2 (preoperative) to 1,691±589/mm2 (12 months postoperative). Rebubbling DMEK was required in three patients (=20%).
Conclusion: DMEK represents a feasible and safe procedure in achieving better functional results compared to DSAEK. Visual acuity and optical quality can be effectively reestablished after unsuccessful primary DSAEK surgery even in patients with long-standing corneal decompensation. Further investigations are required to validate the preliminary clinical findings.
Keywords: DMEK; DSAEK; corneal edema; corneal transplantation.
Conflict of interest statement
Disclosure BA, ASL, and IS have no financial interests. MS reports consultancy for Oculus, Oertli, Santen, and Zeiss. TK reports consultancy and research for Abbott/J&J, Alcon/Novartis, Oculentis, Oculus, Presbia, Schwind, and Zeiss; consultancy for Allergan, Bausch & Lomb, Dompé, Geuder, Med Update, Merck, Rayner, Santen, Staar, Tear Lab, Thea, Thieme, Uni-Med Verlag, and Ziemer; research for Avedro and Hoya; personal fees from Allergan, Bausch & Lomb, Dompé, Geuder, Med Update, Merck, Rayner, Santen, Staar, Tear Lab, Thea, Thieme, Uni-Med, and Ziemer; grants and other from Avedro and Hoya; and grants and personal fees from Carl Zeiss, Johnson&Johnson, Novartis/Alcon, Oculentis, Oculus, Schwind, Presbia, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures

References
-
- Bahar I, Kaiserman I, McAllum P, Slomovic A, Rootman D. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008;115(9):1525–1533. - PubMed
-
- Price FW, Feng MT, Price MO. Evolution of endothelial keratoplasty: where are we headed? Cornea. 2015;34(Supp 10):S41–S47. - PubMed
-
- Marques RE, Guerra PS, Sousa DC, Gonçalves AI, Quintas AM, Rodrigues W. DMEK versus DSAEK for Fuchs’ endothelial dystrophy: a meta-analysis. Eur J Ophthalmol. 2018;29(1):15–22. - PubMed
-
- Pavlovic I, Shajari M, Herrmann E, Schmack I, Lencova A, Kohnen T. Meta-analysis of postoperative outcome parameters comparing Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Cornea. 2017;36(12):1445–1451. - PubMed
-
- Heinzelmann S, Böhringer D, Eberwein P, Reinhard T, Maier P. Outcomes of Descemet membrane endothelial keratoplasty, Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty from a single centre study. Graefes Arch Clin Exp Ophthalmol. 2016;254(3):515–522. - PubMed
LinkOut - more resources
Full Text Sources

