BOS-93, a novel bromophenol derivative, induces apoptosis and autophagy in human A549 lung cancer cells via PI3K/Akt/mTOR and MAPK signaling pathway
- PMID: 30988770
- PMCID: PMC6447907
- DOI: 10.3892/etm.2019.7402
BOS-93, a novel bromophenol derivative, induces apoptosis and autophagy in human A549 lung cancer cells via PI3K/Akt/mTOR and MAPK signaling pathway
Abstract
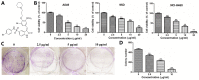
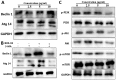
The novel bromophenol derivative, 3-(3-bromo-5-methoxy-4-(3-(piperidin-1-yl)propoxy)benzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide (BOS-93), was synthesized in the CAS Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences (Qingdao, China). Experimental studies have demonstrated that it could induce apoptosis and autophagy in human A549 lung cancer cells, and it could also inhibit tumor growth in human A549 lung cancer xenograft models. In the present study, the molecular pathways underlying these effects were identified. The results demonstrated that BOS-93 could inhibit cell proliferation in A549 cells and block A549 cells at the G0/G1 phase. Furthermore, BOS-93 could induce apoptosis, activate caspase-3 and poly ADP ribose polymerase, and increase the B cell lymphoma (Bcl)-2 associated X protein/Bcl-2 ratio. Notably, BOS-93 could also induce autophagy in A549 cells. BOS-93-induced autophagy was confirmed by detecting light chain 3 (LC3)-I/LC3-II conversion and increasing expression of beclin1 and autophagy-related gene 14. Notably, BOS-93-induced autophagy could be inhibited by the autophagy inhibitor 3-MA. Flow cytometry, transmission electron microscopy (TEM) and western blot analysis indicated that BOS-93 induced apoptosis and autophagy activities by deactivating phosphoinositide 3-kinase/protein kinase B/mechanistic target of rapamycin and activating the mitogen-activated protein kinase signaling pathway. The present findings indicated that BOS-93 might be a novel anti-cancer agent for treatment of human lung cancer.
Keywords: apoptosis; autophagy; bromophenol; phosphoinositide 3-kinase/protein kinase B/mechanistic target of rapamycin signaling; reactive oxygen species.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
