Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView
- PMID: 30988894
- PMCID: PMC6450530
- DOI: 10.1080/20013078.2019.1596016
Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView
Abstract
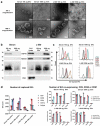
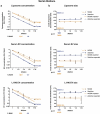
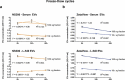
The expanding field of extracellular vesicle (EV) research needs reproducible and accurate methods to characterize single EVs. Nanoparticle Tracking Analysis (NTA) is commonly used to determine EV concentration and diameter. As the EV field is lacking methods to easily confirm and validate NTA data, questioning the reliability of measurements remains highly important. In this regard, a comparison addressing measurement quality between different NTA devices such as Malvern's NanoSight NS300 or Particle Metrix' ZetaView has not yet been conducted. To evaluate the accuracy and repeatability of size and concentration determinations of both devices, we employed comparative methods including transmission electron microscopy (TEM) and single particle interferometric reflectance imaging sensing (SP-IRIS) by ExoView. Multiple test measurements with nanospheres, liposomes and ultracentrifuged EVs from human serum and cell culture supernatant were performed. Additionally, serial dilutions and freeze-thaw cycle-dependent EV decrease were measured to determine the robustness of each system. Strikingly, NanoSight NS300 exhibited a 2.0-2.1-fold overestimation of polystyrene and silica nanosphere concentration. By measuring serial dilutions of EV samples, we demonstrated higher accuracy in concentration determination by ZetaView (% BIAS range: 2.7-8.5) in comparison with NanoSight NS300 (% BIAS range: 32.9-36.8). The concentration measurements by ZetaView were also more precise (% CV range: 0.0-4.7) than measurements by NanoSight NS300 (% CV range: 5.4-10.7). On the contrary, quantitative TEM imaging indicated more accurate EV sizing by NanoSight NS300 (% DTEM range: 79.5-134.3) compared to ZetaView (% DTEM range: 111.8-205.7), while being equally repeatable (NanoSight NS300% CV range: 0.8-6.7; ZetaView: 1.4-7.8). However, both devices failed to report a peak EV diameter below 60 nm compared to TEM and SP-IRIS. Taken together, NTA devices differ strongly in their hardware and software affecting measuring results. ZetaView provided a more accurate and repeatable depiction of EV concentration, whereas NanoSight NS300 supplied size measurements of higher resolution.
Keywords: Extracellular vesicles; accuracy; exosomes; nanoparticle tracking analysis; repeatability; reproducibility; single particle interferometric reflectance imaging sensing; transmission electron microscopy.
Figures



References
-
- van Niel G, D’Angelo G, Raposo G.. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. PubMed PMID: 29339798. - PubMed
-
- Lotvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913 PubMed PMID: 25536934; PubMed Central PMCID: PMCPMC4275645. eng. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

