2D/2D Heterojunctions for Catalysis
- PMID: 30989023
- PMCID: PMC6446599
- DOI: 10.1002/advs.201801702
2D/2D Heterojunctions for Catalysis
Abstract
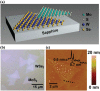
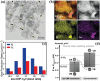
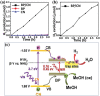
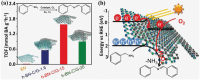
2D layered materials with atomic thickness have attracted extensive research interest due to their unique physicochemical and electronic properties, which are usually very different from those of their bulk counterparts. Heterojunctions or heterostructures based on ultrathin 2D materials have attracted increasing attention due to the integrated merits of 2D ultrathin components and the heterojunction effect on the separation and transfer of charges, resulting in important potential values for catalytic applications. Furthermore, 2D/2D heterostructures with face-to-face contact are believed to be a preferable dimensionality design due to their large interface area, which would contribute to enhanced heterojunction effect. Here, the cutting-edge research progress in 2D/2D heterojunctions and heterostructures is highlighted with a specific emphasis on synthetic strategies, reaction mechanism, and applications in catalysis (photocatalysis, electrocatalysis, and organic synthesis). Finally, the key issues and development perspectives in the applications of 2D/2D layered heterojunctions and heterostructures in catalysis are also discussed.
Keywords: 2D nanojunctions; electrocatalysis; organic synthesis; photocatalysis; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Low J. X., Yu J. G., Jaroniec M., Wageh S., Al‐Ghamdi A. A., Adv. Mater. 2017, 29, 1601694. - PubMed
-
- Wang H. L., Zhang L. S., Chen Z. G., Hu J. Q., Li S. J., Wang Z. H., Liu J. S., Wang X. C., Chem. Soc. Rev. 2014, 43, 5234. - PubMed
-
- Ma X. D., Jiang D. L., Xiao P., Jin Y., Meng S. C., Chen M., Catal. Sci. Technol. 2017, 7, 3481.
-
- Novoselov K. S., Mishchenko A., Carvalho A., Castro Neto A. H., Science 2016, 353, aac9439. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
