Characterization of Tendon-Specific Markers in Various Human Tissues, Tenocytes and Mesenchymal Stem Cells
- PMID: 30989042
- PMCID: PMC6439073
- DOI: 10.1007/s13770-019-00182-2
Characterization of Tendon-Specific Markers in Various Human Tissues, Tenocytes and Mesenchymal Stem Cells
Abstract
Background: Unlike bone, cartilage, or muscle, tendon-specific markers are not well established. The purpose of the study was to investigate expression pattern and level of 6 well-known tendon-specific markers, in various human musculoskeletal tissues, tenocytes, and mesenchymal stem cells (MSCs).
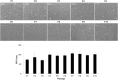
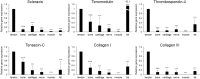
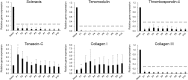
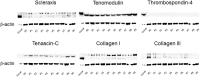
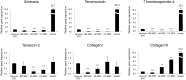
Methods: Musculoskeletal tissue samples of tendon, bone, cartilage, nerve, muscle, and fat were obtained from patients undergoing orthopedic surgery. Tenocytes, MSCs from bone marrow, adipose tissue, and umbilical cord were isolated from each tissue and cultured. Six tendon-specific markers, scleraxis (Scx), tenomodulin (TNMD), thrombospondin-4 (TSP-4), tenascin-C (TNC), type I collagen (Col I), and type III collagen (Col III) were investigated in tendon tissue, tenocytes, and MSCs.
Results: mRNA levels of 6 tendon-specific markers were significantly higher in tendon tissue that in other connective tissues levels of Scx, TNMD, TSP-4, and Col III immediately decreased after plating tenocytes in culture dishes whereas those of TNC and Col I did not. In comparison with tendon tissue, mRNA levels pattern of Scx, TNMD, and TSP-4 in tenocytes were significantly higher than that in MSCs, but lower than in tendon tissue whereas expression pattern of TNC, Col I and III showed different pattern with each other.
Conclusion: This study demonstrated that 6 commonly used tendon-specific markers were mainly expressed in tendon tissue, but that expression level and pattern of the tendon-specific markers with respect to kinds of tissues, culture duration of tenocytes and sources of MSCs.
Keywords: Biomarkers; Mesenchymal stem cells; Scleraxis; Tendons; Thrombospondin-4.
Conflict of interest statement
All authors declare that they have no conflict of interest.The study protocol was approved by the institutional review board at our institution, and was conducted in accordance with the approved guidelines (Seoul National University Boramae Medical Center Institutional Review Board No. 20120405/06-2012-78/118). All patients from whom tissue specimens were harvested provided informed consent.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
