Differentiation Capacity of Monocyte-Derived Multipotential Cells on Nanocomposite Poly(e-caprolactone)-Based Thin Films
- PMID: 30989043
- PMCID: PMC6439045
- DOI: 10.1007/s13770-019-00185-z
Differentiation Capacity of Monocyte-Derived Multipotential Cells on Nanocomposite Poly(e-caprolactone)-Based Thin Films
Abstract
Background: Μonocyte-derived multipotential cells (MOMCs) include progenitors capable of differentiation into multiple cell lineages and thus represent an ideal autologous transplantable cell source for regenerative medicine. In this study, we cultured MOMCs, generated from mononuclear cells of peripheral blood, on the surface of nanocomposite thin films.
Methods: For this purpose, nanocomposite Poly(e-caprolactone) (PCL)-based thin films containing either 2.5 wt% silica nanotubes (SiO2ntbs) or strontium hydroxyapatite nanorods (SrHAnrds), were prepared using the spin-coating method. The induced differentiation capacity of MOMCs, towards bone and endothelium, was estimated using flow cytometry, real-time polymerase chain reaction, scanning electron microscopy and fluorescence microscopy after cells' genetic modification using the Sleeping Beauty Transposon System aiming their observation onto the scaffolds. Moreover, Wharton's Jelly Mesenchymal Stromal Cells were cultivated as a control cell line, while Human Umbilical Vein Endothelial Cells were used to strengthen and accelerate the differentiation procedure in semi-permeable culture systems. Finally, the cytotoxicity of the studied materials was checked with MTT assay.
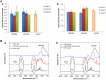
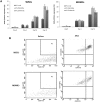
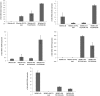
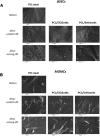
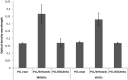
Results: The highest differentiation capacity of MOMCs was observed on PCL/SiO2ntbs 2.5 wt% nanocomposite film, as they progressively lost their native markers and gained endothelial lineage, in both protein and transcriptional level. In addition, the presence of SrHAnrds in the PCL matrix triggered processes related to osteoblast bone formation.
Conclusion: To conclude, the differentiation of MOMCs was selectively guided by incorporating SiO2ntbs or SrHAnrds into a polymeric matrix, for the first time.
Keywords: Monocyte-derived multipotential cells; Poly(ε-caprolactone); Silica nanotubes; Strontium hydroxyapatite nanorods.
Conflict of interest statement
The authors declare that they have no conflict of interest.This study was approved by the Ethics Committee of Aristotle University of Thessaloniki, School of Medicine (390-9/1.7.2017) and University Hospital AXEPA of Thessaloniki (3868/24.1.2018). This research involves Human Participants after their informed consent. Peripheral blood samples were collected from healthy volunteer donors while mesenchymal stromal cells and HUVEC were isolated from Wharton’s jelly after parents approval during stem cell banking in Biohellenika SA Biotechnology Company.
Figures



Similar articles
-
Fabrication of Hydroxyapatite with Bioglass Nanocomposite for Human Wharton's-Jelly-Derived Mesenchymal Stem Cell Growing Substrate.Int J Mol Sci. 2021 Sep 6;22(17):9637. doi: 10.3390/ijms22179637. Int J Mol Sci. 2021. PMID: 34502544 Free PMC article.
-
Effect of topology of poly(L-lactide-co-ε-caprolactone) scaffolds on the response of cultured human umbilical cord Wharton's jelly-derived mesenchymal stem cells and neuroblastoma cell lines.J Biomater Sci Polym Ed. 2014 Jul;25(10):1028-44. doi: 10.1080/09205063.2014.918457. Epub 2014 May 23. J Biomater Sci Polym Ed. 2014. PMID: 24856087
-
Degradation of Poly(ε-caprolactone) and bio-interactions with mouse bone marrow mesenchymal stem cells.Colloids Surf B Biointerfaces. 2018 Mar 1;163:107-118. doi: 10.1016/j.colsurfb.2017.12.039. Epub 2017 Dec 21. Colloids Surf B Biointerfaces. 2018. PMID: 29287231
-
Human-derived extracellular matrix from Wharton's jelly: An untapped substrate to build up a standardized and homogeneous coating for vascular engineering.Acta Biomater. 2017 Jan 15;48:227-237. doi: 10.1016/j.actbio.2016.10.018. Epub 2016 Oct 18. Acta Biomater. 2017. PMID: 27769940
-
Derivation of multipotent progenitors from human circulating CD14+ monocytes.Exp Hematol. 2010 Jul;38(7):557-63. doi: 10.1016/j.exphem.2010.03.015. Epub 2010 Mar 31. Exp Hematol. 2010. PMID: 20362030 Review.
Cited by
-
Influence of spin coating parameters on the fabrication of free standing porous and nonporous poly(ε-caprolactone) based films.Sci Rep. 2025 Jul 11;15(1):25119. doi: 10.1038/s41598-025-10425-z. Sci Rep. 2025. PMID: 40646120 Free PMC article.
-
Composite Membranes of Poly(ε-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications.Molecules. 2019 Aug 23;24(17):3067. doi: 10.3390/molecules24173067. Molecules. 2019. PMID: 31450742 Free PMC article.
-
Enhancement of mesenchymal stem cells' chondrogenic potential by type II collagen-based bioscaffolds.Mol Biol Rep. 2023 Jun;50(6):5125-5135. doi: 10.1007/s11033-023-08461-x. Epub 2023 Apr 28. Mol Biol Rep. 2023. PMID: 37118382 Free PMC article.
References
-
- O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today (Kidlington) 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
