PI3K activation increases SDF-1 production and number of osteoclast precursors, and enhances SDF-1-mediated osteoclast precursor migration
- PMID: 30989092
- PMCID: PMC6449702
- DOI: 10.1016/j.bonr.2019.100203
PI3K activation increases SDF-1 production and number of osteoclast precursors, and enhances SDF-1-mediated osteoclast precursor migration
Abstract
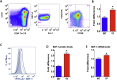
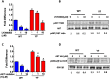
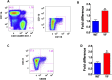
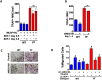
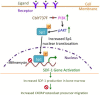
Our previous studies showed that in a mouse model in which PI3K-AKT activation was increased (YF mice), osteoclast numbers and levels of SDF-1, a chemokine, were augmented. The purpose of this study was to delineate the role of PI3K activation in regulating SDF-1 production and examine whether SDF-1 can stimulate differentiation and/or migration of osteoclast precursors. Using flow cytometric analysis, we demonstrated that compared to wild type mice, bone marrow of YF mice had increased numbers of CXCL12 abundant reticular (CAR) cells, that are a major cell type responsible for producing SDF-1. At the molecular level, transcription factor specificity protein 1 (Sp1) induced an increased transcription of SDF-1 that was dependent on PI3K/AKT activation. YF mice also contained an increased number of osteoclast precursors, in which expression of CXCR4, a major receptor for SDF-1, was increased. SDF-1 did not induce differentiation of osteoclast precursors into mature osteoclasts; compared to cells derived from WT mice, cells obtained from YF mice were more responsive to SDF-1. In conclusion, we demonstrate that PI3K activation resulted in increased SDF-1, increased the number of osteoclast precursors, and enhanced osteoclast precursor migration in response to SDF-1.
Keywords: CAR cells; Migration; Osteoclast precursors; PI3K; SDF-1.
Figures

References
-
- Adapala N.S., Barbe M.F., Langdon W.Y., Tsygankov A.Y., Sanjay A. Cbl-phosphatidylinositol 3 kinase interaction differentially regulates macrophage colony-stimulating factor-mediated osteoclast survival and cytoskeletal reorganization. Ann. N. Y. Acad. Sci. 2010;1192:376–384. - PubMed
-
- Arai F., Miyamoto T., Ohneda O., Inada T., Sudo T., Brasel K., Miyata T., Anderson D.M., Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999;190:1741–1754. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

