VPS4 triggers constriction and cleavage of ESCRT-III helical filaments
- PMID: 30989108
- PMCID: PMC6457934
- DOI: 10.1126/sciadv.aau7198
VPS4 triggers constriction and cleavage of ESCRT-III helical filaments
Abstract
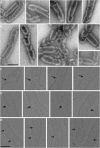
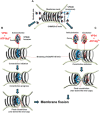
Many cellular processes such as endosomal vesicle budding, virus budding, and cytokinesis require extensive membrane remodeling by the endosomal sorting complex required for transport III (ESCRT-III). ESCRT-III protein family members form spirals with variable diameters in vitro and in vivo inside tubular membrane structures, which need to be constricted to proceed to membrane fission. Here, we show, using high-speed atomic force microscopy and electron microscopy, that the AAA-type adenosine triphosphatase VPS4 constricts and cleaves ESCRT-III CHMP2A-CHMP3 helical filaments in vitro. Constriction starts asymmetrically and progressively decreases the diameter of CHMP2A-CHMP3 tubular structure, thereby coiling up the CHMP2A-CHMP3 filaments into dome-like end caps. Our results demonstrate that VPS4 actively constricts ESCRT-III filaments and cleaves them before their complete disassembly. We propose that the formation of ESCRT-III dome-like end caps by VPS4 within a membrane neck structure constricts the membrane to set the stage for membrane fission.
Figures



References
-
- Scourfield E. J., Martin-Serrano J., Growing functions of the ESCRT machinery in cell biology and viral replication. Biochem. Soc. Trans. 45, 613–634 (2017). - PubMed
-
- Juan T., Fürthauer M., Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 74, 66–77 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

