Phenotypical and functional heterogeneity of neural stem cells in the aged hippocampus
- PMID: 30989815
- PMCID: PMC6612636
- DOI: 10.1111/acel.12958
Phenotypical and functional heterogeneity of neural stem cells in the aged hippocampus
Abstract
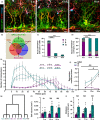
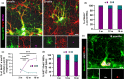
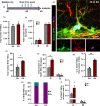
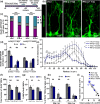
Adult neurogenesis persists in the hippocampus of most mammal species during postnatal and adult life, including humans, although it declines markedly with age. The mechanisms driving the age-dependent decline of hippocampal neurogenesis are yet not fully understood. The progressive loss of neural stem cells (NSCs) is a main factor, but the true neurogenic output depends initially on the actual number of activated NSCs in each given time point. Because the fraction of activated NSCs remains constant relative to the total population, the real number of activated NSCs declines in parallel to the total NSC pool. We investigated aging-associated changes in NSCs and found that there are at least two distinct populations of NSCs. An alpha type, which maintains the classic type-1 radial morphology and accounts for most of the overall NSC mitotic activity; and an omega type characterized by increased reactive-like morphological complexity and much lower probability of division even under a pro-activation challenge. Finally, our results suggest that alpha-type NSCs are able to transform into omega-type cells overtime and that this phenotypic and functional change might be facilitated by the chronic inflammation associated with aging.
Keywords: adult neurogenesis; aging; hippocampus; neural stem cells.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures

References
-
- Bergami, M. , Rimondini, R. , Santi, S. , Blum, R. , Götz, M. , & Canossa, M. (2008). Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety‐like behavior. Proceedings of the National Academy of Sciences of the USA, 105(40), 15570–15575. 10.1073/pnas.0803702105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

