Lactobacillus casei expressing Internalins A and B reduces Listeria monocytogenes interaction with Caco-2 cells in vitro
- PMID: 30989823
- PMCID: PMC6559204
- DOI: 10.1111/1751-7915.13407
Lactobacillus casei expressing Internalins A and B reduces Listeria monocytogenes interaction with Caco-2 cells in vitro
Abstract
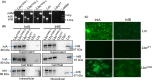
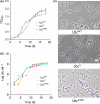
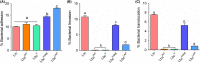
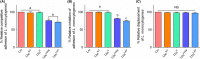
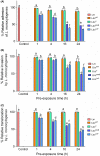
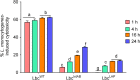
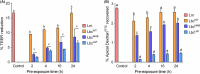
Listeria monocytogenes has been implicated in a number of outbreaks including the recent largest outbreak in South Africa. Current methods for prevention of foodborne L. monocytogenes infection are inadequate, thus raising a need for an alternative strategy. Probiotic bioengineering is considered a prevailing approach to enhance the efficacy of probiotics for targeted control of pathogens. Here, the ability of Lactobacillus casei expressing the L. monocytogenes invasion proteins Internalins A and B (inlAB) to prevent infection was investigated. The inlAB operon was cloned and surface-expressed on L. casei resulting in a recombinant strain, LbcInl AB , and subsequently, its ability to inhibit adhesion, invasion and translocation of L. monocytogenes through enterocyte-like Caco-2 cells was examined. Cell surface expression of InlAB on the LbcInl AB was confirmed by Western blotting and immunofluorescence staining. The LbcInl AB strain showed significantly higher (P < 0.0001) adherence, invasion and translocation of Caco-2 cells than the wild-type L. casei strain (LbcWT ), as well as reduced L. monocytogenes adhesion, invasion and transcellular passage through the cell monolayer than LbcWT . Furthermore, pre-exposure of Caco-2 cells to LbcInl AB significantly reduced L. monocytogenes-induced cell cytotoxicity and epithelial barrier dysfunction. These results suggest that InlAB-expressing L. casei could be a potential practical approach for prevention of listeriosis.
© 2019 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Internalin AB-expressing recombinant Lactobacillus casei protects Caco-2 cells from Listeria monocytogenes-induced damages under simulated intestinal conditions.PLoS One. 2019 Jul 29;14(7):e0220321. doi: 10.1371/journal.pone.0220321. eCollection 2019. PLoS One. 2019. PMID: 31356632 Free PMC article.
-
Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro.PLoS One. 2012;7(1):e29277. doi: 10.1371/journal.pone.0029277. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235279 Free PMC article.
-
Receptor-targeted engineered probiotics mitigate lethal Listeria infection.Nat Commun. 2020 Dec 11;11(1):6344. doi: 10.1038/s41467-020-20200-5. Nat Commun. 2020. PMID: 33311493 Free PMC article.
-
Crossing the Intestinal Barrier via Listeria Adhesion Protein and Internalin A.Trends Microbiol. 2019 May;27(5):408-425. doi: 10.1016/j.tim.2018.12.007. Epub 2019 Jan 17. Trends Microbiol. 2019. PMID: 30661918 Review.
-
Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view.Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a010009. doi: 10.1101/cshperspect.a010009. Cold Spring Harb Perspect Med. 2012. PMID: 23125201 Free PMC article. Review.
Cited by
-
Antibiofilm, AntiAdhesive and Anti-Invasive Activities of Bacterial Lysates Extracted from Pediococcus acidilactici against Listeria monocytogenes.Foods. 2022 Sep 21;11(19):2948. doi: 10.3390/foods11192948. Foods. 2022. PMID: 36230024 Free PMC article.
-
A Listeria ivanovii balanced-lethal system may be a promising antigen carrier for vaccine construction.Microb Biotechnol. 2022 Nov;15(11):2831-2844. doi: 10.1111/1751-7915.14137. Epub 2022 Sep 7. Microb Biotechnol. 2022. PMID: 36069650 Free PMC article.
-
Factors Affecting Spontaneous Endocytosis and Survival of Probiotic Lactobacilli in Human Intestinal Epithelial Cells.Microorganisms. 2022 May 31;10(6):1142. doi: 10.3390/microorganisms10061142. Microorganisms. 2022. PMID: 35744660 Free PMC article.
-
Advanced probiotics: bioengineering and their therapeutic application.Mol Biol Rep. 2024 Feb 25;51(1):361. doi: 10.1007/s11033-024-09309-8. Mol Biol Rep. 2024. PMID: 38403783 Review.
-
Screening, Safety Evaluation, and Mechanism of Two Lactobacillus fermentum Strains in Reducing the Translocation of Staphylococcus aureus in the Caco-2 Monolayer Model.Front Microbiol. 2020 Sep 16;11:566473. doi: 10.3389/fmicb.2020.566473. eCollection 2020. Front Microbiol. 2020. PMID: 33042071 Free PMC article.
References
-
- Aguilar, C. , Vanegas, C. , and Klotz, B. (2011) Antagonistic effect of Lactobacillus strains against Escherichia coli and Listeria monocytogenes in milk. J Dairy Res 78: 136–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

