Phantom-based evaluation of near-infrared intracranial hematoma detector performance
- PMID: 30989838
- PMCID: PMC6989771
- DOI: 10.1117/1.JBO.24.4.045001
Phantom-based evaluation of near-infrared intracranial hematoma detector performance
Abstract
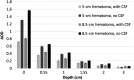
Near-infrared spectroscopy (NIRS) is emerging as a rapid, low-cost approach for point-of-care triage of hematomas resulting from traumatic brain injury. However, there remains a lack of standardized test methods for benchtop performance assessment of these devices and incomplete understanding of relevant light-tissue interactions. We propose a phantom-based test method for systems operating near the 800-nm oxy-/deoxy-hemoglobin isosbestic point and implement it to evaluate a clinical system. Semi-idealized phantom geometries are designed to represent epidural/subdural, subarachnoid, and intracerebral hemorrhages. Measurements of these phantoms are made with a commercial NIRS-based hematoma detector to quantify the effect of hematoma type, depth, and size, as well as measurement repeatability and detector positioning relative to the hematoma. Results indicated high sensitivity to epidural/subdural and subarachnoid hematomas. Intracerebral hematomas are detectable to a maximum depth of ∼2.5 cm, depending on thickness and diameter. The maximum lateral detection area for the single-emitter/single-collector device studied here appears elliptical and decreases strongly with inclusion depth. Overall, this study provides unique insights into hematoma detector function and indicates the utility of modular polymer tissue phantoms in performance tests for emerging NIRS-based cerebral diagnostic technology.
Keywords: hematoma detection; light–tissue interaction; near-infrared spectroscopy; standardization; tissue phantoms; traumatic brain injury.
Figures









Similar articles
-
Performance of a new portable near-infrared spectroscopy device for detection of traumatic intracranial hematoma.Injury. 2023 May;54(5):1278-1286. doi: 10.1016/j.injury.2023.03.014. Epub 2023 Mar 12. Injury. 2023. PMID: 36934009
-
The accuracy of near-infrared spectroscopy in detection of subdural and epidural hematomas.J Trauma. 2006 Dec;61(6):1480-3. doi: 10.1097/01.ta.0000197616.10279.48. J Trauma. 2006. PMID: 17159695 Clinical Trial.
-
Early diagnosis of traumatic intracranial hematomas.J Biomed Opt. 2019 Feb;24(5):1-10. doi: 10.1117/1.JBO.24.5.051411. J Biomed Opt. 2019. PMID: 30719879 Free PMC article. Review.
-
Diagnostic properties of a portable near-infrared spectroscopy to detect intracranial hematoma in traumatic brain injury patients.Eur J Radiol Open. 2020 Jul 29;7:100246. doi: 10.1016/j.ejro.2020.100246. eCollection 2020. Eur J Radiol Open. 2020. PMID: 32775555 Free PMC article.
-
Study of near infrared technology for intracranial hematoma detection.J Biomed Opt. 2000 Apr;5(2):206-13. doi: 10.1117/1.429988. J Biomed Opt. 2000. PMID: 10938785 Review.
Cited by
-
Intracranial Traumatic Hematoma Detection in Children Using a Portable Near-infrared Spectroscopy Device.West J Emerg Med. 2021 Mar 24;22(3):782-791. doi: 10.5811/westjem.2020.11.47251. West J Emerg Med. 2021. PMID: 34125061 Free PMC article.
-
Detectability of low-oxygenated regions in human muscle tissue using near-infrared spectroscopy and phantom models.Biomed Opt Express. 2022 Nov 2;13(12):6182-6195. doi: 10.1364/BOE.473563. eCollection 2022 Dec 1. Biomed Opt Express. 2022. PMID: 36589557 Free PMC article.
-
Continuous monitoring method of cerebral subdural hematoma based on MRI guided DOT.Biomed Opt Express. 2020 May 11;11(6):2964-2975. doi: 10.1364/BOE.388059. eCollection 2020 Jun 1. Biomed Opt Express. 2020. PMID: 32637235 Free PMC article.
-
Optical Properties and Fluence Distribution in Rabbit Head Tissues at Selected Laser Wavelengths.Materials (Basel). 2022 Aug 18;15(16):5696. doi: 10.3390/ma15165696. Materials (Basel). 2022. PMID: 36013828 Free PMC article.
References
-
- Silver J., McAllister T., Yudofsky S., Textbook of Traumatic Brain Injury, American Psychiatric Publishing, New York: (2005).

