Effects of terminal galactose residues in mannose α1-6 arm of Fc-glycan on the effector functions of therapeutic monoclonal antibodies
- PMID: 30990348
- PMCID: PMC6601563
- DOI: 10.1080/19420862.2019.1608143
Effects of terminal galactose residues in mannose α1-6 arm of Fc-glycan on the effector functions of therapeutic monoclonal antibodies
Abstract
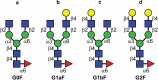
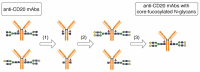
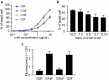
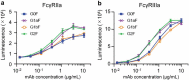
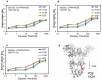
Typical crystallizable fragment (Fc) glycans attached to the CH2 domain in therapeutic monoclonal antibodies (mAbs) are core-fucosylated and asialo-biantennary complex-type glycans, e.g., G2F (full galactosylation), G1aF (terminal galactosylation on the Man α1-6 arm), G1bF (terminal galactosylation on the Man α1-3 arm), and G0F (non-galactosylation). Terminal galactose (Gal) residues of Fc-glycans are known to influence effector functions such as antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity (CDC), but the impact of the G1F isomers (G1aF and G1bF) on the effector functions has not been reported. Here, we prepared four types of glycoengineered anti-CD20 mAbs bearing homogeneous G2F, G1aF, G1bF, or G0F (G2F mAb, G1aF mAb, G1bF mAb, or G0F mAb, respectively), and evaluated their biological activities. Interestingly, G1aF mAb showed higher C1q- and FcγR-binding activities, CDC activity, and FcγR-activation property than G1bF mAb. The activities of G1aF mAb and G1bF mAb were at the same level as G2F mAb and G0F mAb, respectively. Hydrogen-deuterium exchange/mass spectrometry analysis of dynamic structures of mAbs revealed the greater involvement of the terminal Gal residue on the Man α1-6 arm in the structural stability of the CH2 domain. Considering that mAbs interact with FcγR and C1q via their hinge proximal region in the CH2 domain, the structural stabilization of the CH2 domain by the terminal Gal residue on the Man α1-6 arm of Fc-glycans may be important for the effector functions of mAbs. To our knowledge, this is the first report showing the impact of G1F isomers on the effector functions and dynamic structure of mAbs. Abbreviations: ABC, ammonium bicarbonate solution; ACN, acetonitrile; ADCC, antibody-dependent cell-mediated cytotoxicity; C1q, complement component 1q; CDC, complement-dependent cytotoxicity; CQA, critical quality attribute; Endo, endo-β-N-acetylglucosaminidase; FA, formic acid; Fc, crystallizable fragment; FcγR, Fcγ receptors; Fuc, fucose; Gal, galactose; GlcNAc, N-acetylglucosamine; GST, glutathione S-transferase; HER2, human epidermal growth factor receptor 2; HDX, hydrogen-deuterium exchange; HILIC, hydrophilic interaction liquid chromatography; HLB-SPE, hydrophilic-lipophilic balance-solid-phase extraction; HPLC, high-performance liquid chromatography; mAb, monoclonal antibody; Man, mannose; MS, mass spectrometry; PBS, phosphate-buffered saline; SGP, hen egg yolk sialylglycopeptides.
Keywords: Therapeutic monoclonal antibody; antibody-dependent cell-mediated cytotoxicity; complement-dependent cytotoxicity; galactose; glycoengineering.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
