Cullin-3 dependent deregulation of ACTN1 represents a new pathogenic mechanism in nemaline myopathy
- PMID: 30990797
- PMCID: PMC6542616
- DOI: 10.1172/jci.insight.125665
Cullin-3 dependent deregulation of ACTN1 represents a new pathogenic mechanism in nemaline myopathy
Abstract
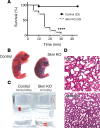
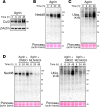
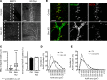
Nemaline myopathy is a congenital neuromuscular disorder characterized by muscle weakness, fiber atrophy and presence of nemaline bodies within myofibers. However, the understanding of underlying pathomechanisms is lacking. Recently, mutations in KBTBD13, KLHL40 and KLHL41, three substrate adaptors for the E3-ubiquitin ligase Cullin-3, have been associated with early-onset nemaline myopathies. We hypothesized that deregulation of Cullin-3 and its muscle protein substrates may be responsible for the disease development. Using Cullin-3 knockout mice, we identified accumulation of non-muscle alpha-Actinins (ACTN1 and ACTN4) in muscles of these mice, which we also observed in KBTBD13 patients. Our data reveal that proper regulation of Cullin-3 activity and ACTN1 levels is essential for normal muscle and neuromuscular junction development. While ACTN1 is naturally downregulated during myogenesis, its overexpression in C2C12 myoblasts triggered defects in fusion, myogenesis and acetylcholine receptor clustering; features that we characterized in Cullin-3 deficient mice. Taken together, our data highlight the importance for Cullin-3 mediated degradation of ACTN1 for muscle development, and indicate a new pathomechanism for the etiology of myopathies seen in Cullin-3 knockout mice and nemaline myopathy patients.
Keywords: Mouse models; Muscle Biology; Skeletal muscle; Ubiquitin-proteosome system.
Conflict of interest statement
Figures








References
-
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6(1):9–20. - PubMed

