Ectonucleotidase tri(di)phosphohydrolase-1 (ENTPD-1) disrupts inflammasome/interleukin 1β-driven venous thrombosis
- PMID: 30990798
- PMCID: PMC6597243
- DOI: 10.1172/JCI124804
Ectonucleotidase tri(di)phosphohydrolase-1 (ENTPD-1) disrupts inflammasome/interleukin 1β-driven venous thrombosis
Abstract
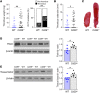
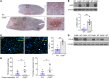
Deep vein thrombosis (DVT), caused by alterations in venous homeostasis is the third most common cause of cardiovascular mortality; however, key molecular determinants in venous thrombosis have not been fully elucidated. Several lines of evidence indicate that DVT occurs at the intersection of dysregulated inflammation and coagulation. The enzyme ectonucleoside tri(di)phosphohydrolase (ENTPD1, also known as CD39) is a vascular ecto-apyrase on the surface of leukocytes and the endothelium that inhibits intravascular inflammation and thrombosis by hydrolysis of phosphodiester bonds from nucleotides released by activated cells. Here, we evaluated the contribution of CD39 to venous thrombosis in a restricted-flow model of murine inferior vena cava stenosis. CD39-deficiency conferred a >2-fold increase in venous thrombogenesis, characterized by increased leukocyte engagement, neutrophil extracellular trap formation, fibrin, and local activation of tissue factor in the thrombotic milieu. This was orchestrated by increased phosphorylation of the p65 subunit of NFκB, activation of the NLRP3 inflammasome, and interleukin-1β (IL-1β) release in CD39-deficient mice. Substantiating these findings, an IL-1β-neutralizing antibody attenuated the thrombosis risk in CD39-deficient mice. These data demonstrate that IL-1β is a key accelerant of venous thrombo-inflammation, which can be suppressed by CD39. CD39 inhibits in vivo crosstalk between inflammation and coagulation pathways, and is a critical vascular checkpoint in venous thrombosis.
Keywords: Cardiovascular disease; Inflammation; Innate immunity; Thrombosis; Vascular Biology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL131993/HL/NHLBI NIH HHS/United States
- T32 HL007853/HL/NHLBI NIH HHS/United States
- K99 HL136784/HL/NHLBI NIH HHS/United States
- R03 AR072107/AR/NIAMS NIH HHS/United States
- L30 HL129373/HL/NHLBI NIH HHS/United States
- R01 NS087147/NS/NINDS NIH HHS/United States
- R01 GM105671/GM/NIGMS NIH HHS/United States
- R01 HL127151/HL/NHLBI NIH HHS/United States
- K08 AR066569/AR/NIAMS NIH HHS/United States
- K23 HL119623/HL/NHLBI NIH HHS/United States
- R01 HL114405/HL/NHLBI NIH HHS/United States
- R01 HL134846/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

