Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala
- PMID: 30990816
- PMCID: PMC6467366
- DOI: 10.1371/journal.pbio.2006421
Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala
Abstract
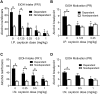
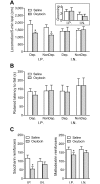
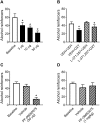
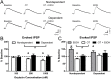
Oxytocin administration has been reported to decrease consumption, withdrawal, and drug-seeking associated with several drugs of abuse and thus represents a promising pharmacological approach to treat drug addiction. We used an established rat model of alcohol dependence to investigate oxytocin's effects on dependence-induced alcohol drinking, enhanced motivation for alcohol, and altered GABAergic transmission in the central nucleus of the amygdala (CeA). Intraperitoneal oxytocin administration blocked escalated alcohol drinking and the enhanced motivation for alcohol in alcohol-dependent but not nondependent rats. Intranasal oxytocin delivery fully replicated these effects. Intraperitoneal administration had minor but significant effects of reducing locomotion and intake of non-alcoholic palatable solutions, whereas intranasal oxytocin administration did not. In dependent rats, intracerebroventricular administration of oxytocin or the oxytocin receptor agonist PF-06655075, which does not cross the blood-brain barrier (i.e., it would not diffuse to the periphery), but not systemic administration of PF-06655075 (i.e., it would not reach the brain), decreased alcohol drinking. Administration of a peripherally restricted oxytocin receptor antagonist did not reverse the effect of intranasal oxytocin on alcohol drinking. Ex vivo electrophysiological recordings from CeA neurons indicated that oxytocin decreases evoked GABA transmission in nondependent but not in dependent rats, whereas oxytocin decreased the amplitude of spontaneous GABAergic responses in both groups. Oxytocin blocked the facilitatory effects of acute alcohol on GABA release in the CeA of dependent but not nondependent rats. Together, these results provide converging evidence that oxytocin specifically and selectively blocks the enhanced motivation for alcohol drinking that develops in alcohol dependence likely via a central mechanism that may result from altered oxytocin effects on CeA GABA transmission in alcohol dependence. Neuroadaptations in endogenous oxytocin signaling may provide a mechanism to further our understanding of alcohol use disorder.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organisation. Global status report on alcohol and health. Geneva, Switzerland: World Health Organization; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

