Peri-Infarct Upregulation of the Oxytocin Receptor in Vascular Dementia
- PMID: 30990880
- PMCID: PMC6467199
- DOI: 10.1093/jnen/nlz023
Peri-Infarct Upregulation of the Oxytocin Receptor in Vascular Dementia
Abstract
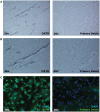
Vascular dementia (VaD) is cognitive decline linked to reduced cerebral blood perfusion, yet there are few therapeutic options to protect cognitive function following cerebrovascular accidents. The purpose of this study was to profile gene expression changes unique to VaD to identify and characterize disease relevant changes that could offer clues for future therapeutic direction. Microarray-based profiling and validation studies of postmortem frontal cortex samples from VaD, Alzheimer disease, and age-matched control subjects revealed that the oxytocin receptor (OXTR) was strongly and differentially upregulated in VaD. Further characterization in fixed tissue from the same cases showed that OXTR upregulation occurs de novo around and within microinfarcts in peri-infarct reactive astrocytes as well as within vascular profiles, likely on microvascular endothelial cells. These results indicate that increased OXTR expression in peri-infarct regions may be a specific response to microvascular insults. Given the established OXTR signaling cascades that elicit antioxidant, anti-inflammatory, and pro-angiogenic responses, the present findings suggest that de novo OXTR expression in the peri-infarct space is a tissue-protective response by astroglial and vascular cells in the wake of ischemic damage that could be exploited as a therapeutic option for the preservation of cognition following cerebrovascular insults.
Keywords: Alzheimer disease; Astrocvytes; Microinfarcts; Microvascular endothelial cells; Oxytocin receptor; Vascular dementia.
© 2019 American Association of Neuropathologists, Inc. All rights reserved.
Figures






References
-
- World Health Organization. Towards a Dementia Plan: A WHO Guide [Internet]. Dementia: A Public Health Priority – World Health Organization; 2017
-
- Wimo A, Jönsson L, Bond J, et al. The worldwide economic impact of dementia 2010. Alzheimer’s Dement 2013;9:1–11 - PubMed
-
- Jorm AF, Jolley D.. The incidence of dementia: A meta-analysis. Neurology 1998;51:728–33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

