Concerted Nucleophilic Aromatic Substitution Reactions
- PMID: 30990931
- PMCID: PMC6899550
- DOI: 10.1002/anie.201902216
Concerted Nucleophilic Aromatic Substitution Reactions
Abstract
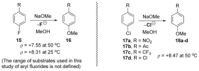
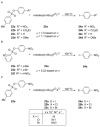
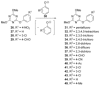
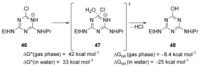
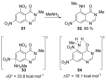
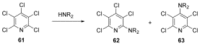
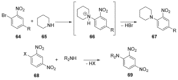
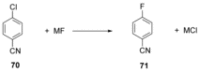
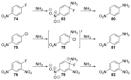
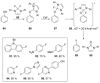
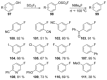
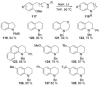
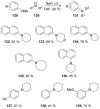
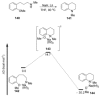
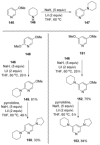
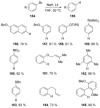
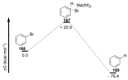
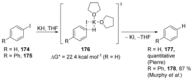
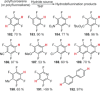
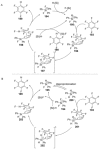
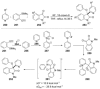
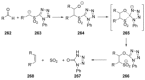
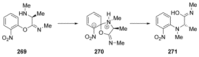
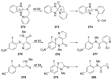
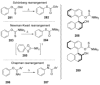
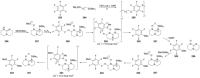
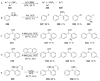
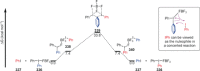
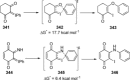
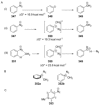
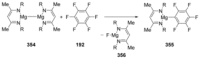
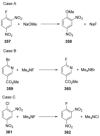
Recent developments in experimental and computational chemistry have identified a rapidly growing class of nucleophilic aromatic substitutions that proceed by concerted (cSN Ar) rather than classical, two-step, SN Ar mechanisms. Whereas traditional SN Ar reactions require substantial activation of the aromatic ring by electron-withdrawing substituents, such activating groups are not mandatory in the concerted pathways.
Keywords: Meisenheimer complex; cSNAr mechanism; concerted reactions; nucleophilic aromatic substitution.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



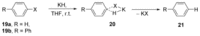

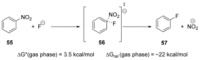
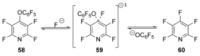




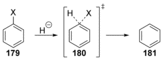


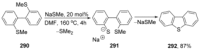


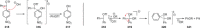
References
-
- Smyth M. B., in March's Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 7 th ed., Wiley, Hoboken, 2013, Chap. 11, pp. 569–641, e-book ISBN: 9781118472217.
-
- Bunnett J. F., Zahler R. E., Chem. Rev. 1951, 49, 273–412.
-
- Terrier F., Modern Nucleophilic Aromatic Substitution, Wiley-VCH, Weinheim, 2013, e-book ISBN: 9783527656141.
-
- Błaziak K., Danikiewicz W., Mąkosza M., J. Am. Chem. Soc. 2016, 138, 7276–7281, and references therein. - PubMed
-
- Takikawa H., Nishii A., Sakai T., Suzuki K., Chem. Soc. Rev. 2018, 47, 8030–8056. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

